PHMB Eye drops for treating Acanthamoeba ocular infections
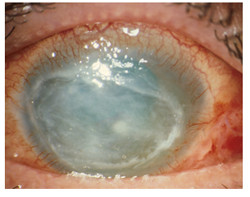
Acanthamoeba keratitis (AK) is a rare parasitic infectious disease of the eye affecting mainly individuals who wear disposable contact lenses. If not treated promptly, it can lead to visual impairment and even blindness. As a result, it is of primary importance to develop and establish efficacious treatment for AK. With this in mind, the EU-funded 'Orphan drug for Acanthamoeba keratitis' (ODAK) project set out to test the efficacy of the drug Polihexanide (PHMB). PHMB has been designated as an orphan drug in the EU for the treatment of AK. During ODAK it will undergo non-clinical as well as Phase I/Phase III clinical evaluation. Apart from testing the quality, safety and efficacy of PHMB, ODAK partners will also provide recommendations for the clinical practice and management of AK. Retrospective evaluation of the clinical outcomes of AK patients treated with unlicensed drugs indicated that 96 % of patients were using contact lenses, and that PHMB was the most frequently used medication. The efficacy of PHMB established it as a promising candidate for obtaining an official AK treatment licence. ODAK partners therefore set out to optimise formulation of the PHMB active ingredient. Different formulations have so far been tested in terms of efficacy and ocular tolerance in pre-clinical models. At the same time, the consortium is engaging in dissemination activities to raise awareness about the disease, its diagnosis and treatment. The project has also been promoted on social media, providing information on its activities and progress. By improving the current empirical treatment of AK with PHMB, ODAK hopes to increase drug bioavailability at the ocular surface and hence its therapeutic index. The envisioned eye drops formulation is expected to also minimise side-effects as well as the need for hospitalisation and surgery.
Keywords
Eye infection, contact lenses, Acanthamoeba keratitis, Polihexanide, PHMB, eye drop