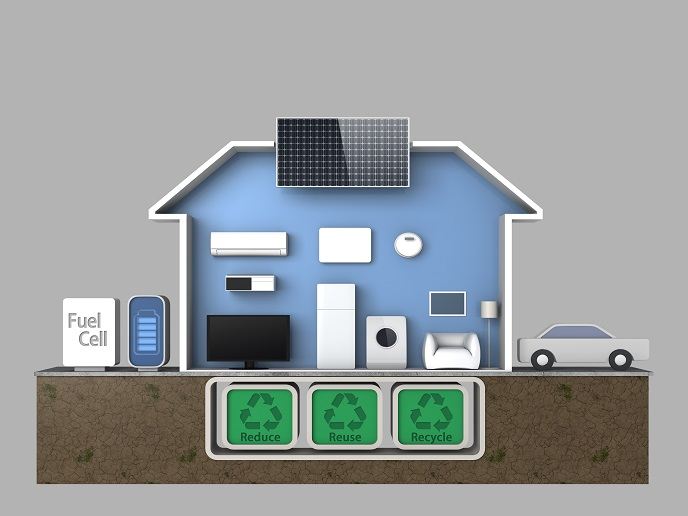
Bioelectricity for alternative energy
Bioelectrochemical systems are electrochemical devices that convert chemical energy to electricity. Purified enzymes catalyse the oxidation of fuel at the anode electrode and carry out the reduction of the electron acceptor at the cathode electrode. However, these enzymes require pure substrate, which at present is not economical for large-scale applications. This problem was addressed by the ELECTROENZEQUEST (Bioelectrochemical system for enzyme catalyzed CO2 sequestration for the recovery of commercially viable carbonated water and methanol) project, which explored the use of carbon dioxide (CO2) as a substrate. The project investigated the mechanisms behind the sequestration of atmospheric CO2 through enzyme cocktails, which used multiple enzymes together to harness bioelectricity. CO2 was considered for both anodoxic oxidation and cathodic reduction in order to obtain carbonated water and methanol, respectively. Researchers immobilised carbonic anhydrase (CA) onto the electrode and evaluated its use as an anode for electrogenesis (the generation of electricity by living organisms). They carried out bioelectrochemical analysis of the immobilised CA cathode and optimised the various factors influencing the role of CA in CO2 sequestration. Scientists immobilised formate dehydrogenase (Fate DH), formaldehyde dehydrogenase (Fald DH) and alcohol dehydrogenase on an electrode. This was introduced as a cathode in the operating CA fuel cell. They also optimised the performance of nicotinamide adenine dinucleotide and pyrroloquinoline quinone in the fuel cell, which mediated the electron transfer from electrode to the substrate and the conversion of CO2 to methanol during cathodic reduction. In addition, researchers studied the feasibility of converting CO2 into formic acid using Fate DH in free form, before immobilising it onto the graphite-based VITO-CoRETM electrode. They then successfully immobilised all three enzymes onto the graphite-based VITO-CoRETM electrode for the production of methanol. CA was then added at a later stage to increase productivity. Further experiments were carried out using the three enzymes together on the electrode, where ethanol production was observed at the rate of 0.6 kg per cubic metre per hour. However, when Fald DH was excluded, there was no decrease in the productivity of ethanol. Although ethanol was produced rather than methanol, ethanol can be produced directly from formic acid, which is economically more viable. ELECTROENZEQUEST therefore highlighted the importance of CO2 sequestration for mitigating the impacts of climate change and the need for alternative biofuels.