Improved materials for tissue engineering
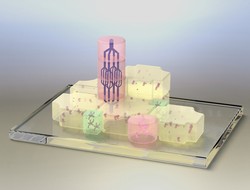
Each tissue has different cell types that require distinct environmental cues to develop properly. When preparing a tissue graft for transplantation purposes, an appropriate controlled environment is required for cells to differentiate and integrate into the host tissue. The EU-funded PREVASCIN project proposed to improve the integration of engineered tissue into the host by including in the graft a neural and vascular network. Towards this goal, the project used the ‘Living Legos’ building block system combined with matrix elasticity and local growth factor delivery to control the formation of separate tissue components. Apart from control of tissue development, this strategy allowed for the vascularisation and innervation of the engineered graft from a single cell source. Researchers designed a composite hydrogel system with a chemical modification of gelatin and polyethylene glycol dimethacrylate (PEGDMA). Varying this PEGDMA content permitted the fine tuning of the mechanical properties of the system. The capacity of this system to support differentiation of human mesenchymal stromal cells (MSC) was then investigated. Results showed differentiation down the neural and osteogenic lineages. Apart from this, an aptamer-based technology, which enabled the spatiotemporal distribution of growth factors inside hydrogels was developed. In particular, the inclusion of vascular endothelial growth factor (VEGF) within the hydrogel supported the specific organisation of endothelial cells. The PREVASCIN tissue building block approach allowed scientists to prepare tissue constructs containing regions with different hydrogel compositions. Researchers investigated the generation of bone tissue with neural structures and bone tissue with endothelial structures. Although vascular and neural networks were not developed fully and remain to be further investigated, the strategy is highly flexible and can be easily translated to other applications and engineered tissues. Overall, the PREVASCIN study addressed an important bottleneck in the field of tissue engineering regarding the correct integration of transplanted tissue into the host. Future optimisation efforts are expected to improve this modular approach for eventual clinical implementation.