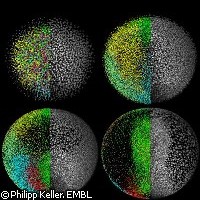
New insights into embryonic development
EU-funded research conducted at the European Molecular Biology Laboratory (EMBL) has helped scientists gain new insights into embryonic development. Thanks to a newly developed microscope, they were able to follow the first 24 hours in the life of a zebrafish from the single cell stage to 20,000 cells. On the basis of the data gleaned through this observation, Philipp Keller and colleagues at EMBL have generated a three-dimensional digital representation of a zebrafish embryo. Their findings were recently published in Science magazine. 'The digital embryo is like Google EarthTM for embryonic development. It gives an overview of everything that happens in the first 24 hours and allows you to zoom in on all cellular and even subcellular details,' says Dr Joachim Wittbrodt, formerly of the EMBL. Using 'digital scanned laser light sheet fluorescence' microscopy, the researchers were able to watch cell nuclei localisation and movement during those first few crucial hours of embryonic development in extraordinary detail. The microscope scans the living organism from different directions using a sheet of light. Computers can then compile the data to create a three-dimensional image. More specifically, the microscopic scan provided in vivo images of the zebrafish embryo at 1.5 billion voxels (the three-dimensional counterpart to pixels) per minute. 'Imagine following all inhabitants of a town over the course of one day using a telescope in space. This comes close to tracking the tens of thousands of cells that make up a vertebrate embryo - only that the cells move in three dimensions,' explains Philipp Keller. Together with Annette Schmidt he carried out the research in the labs of Dr Wittbrodt at EMBL. The scientists' observations are unprecedented in as complex an organism as a vertebrate, and show that the fundamental movements of cells that later form the heart and other organs are different than previously assumed. Furthermore, they found that it is signals from the maternal side of the genome that induce the initial morphodynamic symmetry break early on in development. This process determines the head-tail body axis of the fish. Observing cell development and recording all their trajectories 'is extremely important if you eventually want to correlate gene function with morphogenetic output,' explains Dr Wittbrodt. 'So, for that reason, I feel that it's crucial that we are now able to, on one hand, map all the movements of the cells and eventually - and this is the long-term perspective - correlate that with the genome and all the genomic information that we have.' The zebrafish is a freshwater fish that has a nearly transparent body during the early stages of its development. This special trait enables unique visual access to the animal's internal anatomy and makes zebrafish model organisms highly valued in the study of embryonic development, toxicology and toxicopathology, specific gene function and roles of signalling pathways. The scientists are now expanding the use of their new technique to develop digital versions of other model organisms such as molluscs, chicken embryos and others. Their results will be made available to the general public as well as the scientific community in order to help in science teaching and research. Part of the financing for the study came from the PLURIGENES ('Pluripotency associated genes to de-differentiate neural cells into pluripotent cells') project, which is funded under the Life Science Theme of the European Commission's Sixth Framework Programme (FP6).