3-D structure of mammalian sperm receptor revealed
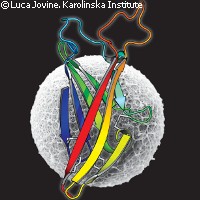
Scientists at the Karolinska Institute in Sweden have uncovered the three-dimensional (3-D) structure of a protein directly involved in egg-sperm binding. The findings have important implications for human reproductive medicine and may be used to develop novel non-hormonal contraceptives. The study, funded in part by the EU through a Marie Curie European Reintegration Grant, is published in the journal Nature. The mammalian egg coat, called the zona pellucida (ZP), plays a major role in fertilisation and prevents more than one sperm from entering the egg. ZP proteins in mice, called ZP2 and ZP3, act as sperm receptors during fertilisation. They contain a common sequence that allows them to form the egg coat - a matrix of filaments that completely surrounds the egg. ZP proteins have a region called the ZP domain, and mutations in this domain can lead to severe disorders including infertility, deafness and some cancers. The exact structure of proteins containing the ZP domain has been very difficult to obtain due to their dynamic nature. Because of its structural complexity, studying its amino-acid sequence alone would not be enough to adequately describe the unique features of the ZP domain. 'ZP3 was identified almost 30 years ago, but obtaining structural information on this key reproductive protein has been technically challenging due to its high heterogeneity,' explained lead investigator Luca Jovine. In this latest research, scientists described a high-resolution, 3-D structure of a key part of ZP3 using protein crystallography. In revealing its structure in unprecedented detail, the researchers showed that a part of its ZP domain, ZP-N, folds in a novel way reminiscent of (but quite distinct from) antibodies. According to the study, the findings demonstrate that ZP-N is a domain of its own, and is a distinct subtype of the immunoglobulin superfamily. The immunoglobulin superfamily (IgSF) is a group of proteins involved in the recognition, binding, and adhesion processes of cells. Members share certain structural features with antibodies, including a domain known as an immunoglobulin domain (or fold). These domains have a sandwich-like structure formed by two sheets of beta strands. The researchers found that two thirds of ZP-N consists of beta-strands interconnected by loops of varying lengths and shapes to form a sandwich, similar to that found in immunoglobulin proteins but with distinctive features. The researchers believe their methods can be used in future studies to characterise a region of ZP3, which is important for polymerisation. Such an achievement would significantly improve our understanding of human infertility. It might also lead to the development of novel targeted, non-hormonal contraceptives. 'Mammalian fertilisation involves a highly complex series of events. Our findings pave the way for future investigations into this fascinating subject by providing a first snapshot of the beginning of life at atomic resolution,' said Dr Jovine. The description of ZP-N has also provided insights into the molecular basis of ZP-related human diseases. 'The structure of ZP3 ZP-N offers a first glimpse into the basis of mammalian fertilisation at atomic resolution,' the study concludes. 'Because many pathological mutations in other ZP domain proteins fall within ZP-N, this work will not only be relevant for reproductive medicine, but also for severe human diseases such as non-syndromic deafness or renal and vascular disorders.'
Countries
Sweden