New technologies create chemical products out of thin air
The EU-funded CELBICON(opens in new window) project addressed this challenge, capturing CO2 from the atmosphere and converting it by a sequence of electrochemical and biochemical steps into complex, valuable chemicals. These have an extremely low or even negative carbon footprint and the energy required for the conversions can ultimately come from sustainable energy sources. This will help the EU to meet target levels of 20 % reduction in greenhouse gases(opens in new window) by 2020, and that further reduction up to 80-95 % by 2050(opens in new window) compared to 1990 is achieved. Project partners developed technologies to realise unprecedented yield and efficiency results in a high-pressure and a low-pressure processing line. “The first produced polyhydroxyalkanoate (PHA) bioplastic and pressurised methane (CH4) via intermediate electrochemical generation of pressurised syngas followed by specific fermentation steps. The second processing line focused on the production of value-added chemicals by fermentation of CO2-reduction water-soluble C1 (one-carbon molecule) intermediates,” says project coordinator Debora Fino. Both process lines underwent a thorough component development R&D programme, assembling three test rigs to the stage where they could be commercialised. “These involved direct CO2 capture from air, an energy-efficient method to compress and dissolve CO2 in water to obtain high-pressure concentrated CO2 solutions, and finally the biochemical production of CH4 from CO2 and hydrogen,” Fino explains.
An innovative approach
The first CO2 capture technology developed by consortium members has already been completely integrated into a carbon capture and utilisation process. This approach exploits two prospective renewable electricity-driven electrochemical processes aimed at converting the atmospheric CO2 to useful chemicals. The second functional and automated prototype for CO2 compression and dissolution achieved around a 40 % reduction in energy demand compared to the standard process (first gas compression and then dissolution at the high-pressure level). The proposed concept entails the use of a dense water spray within the compressor, to simultaneously cool the compressed gas. This results in isothermal conditions along with immediate dissolution of the CO2 in the water, as the pressure increases due to the large interface between the CO2 and the water droplets and filaments. “The result is a process that reduces the overall energy consumption due to the isothermal conditions and the simultaneous reduction of the amount of CO2 in the gaseous phase to be compressed,” Fino reports.
Greater efficiency, lower costs
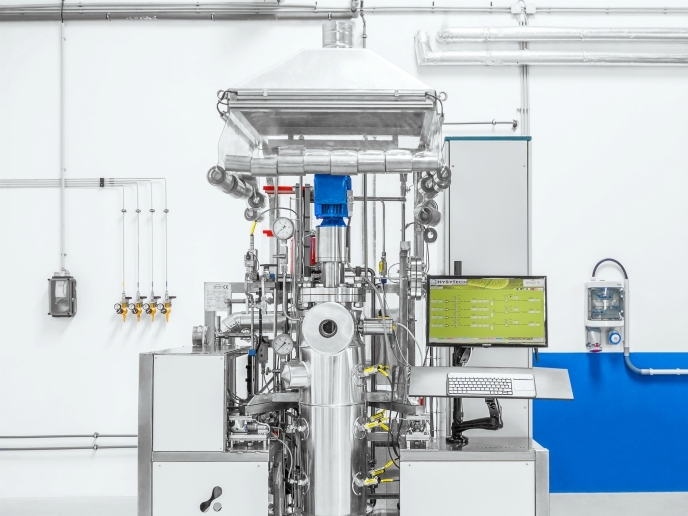
Finally, the third biochemical process achieved CO2 conversion to CH4 in the presence of hydrogen and carbon monoxide generated in an electrolyser in a custom integrated pressure-resistant bioreactor skid. The bioreactor is designed and manufactured to be operated at ambient pressure of 50 barg gauge pressure. In addition, new screening strategies for gas-converting bioprocess development were established and multiple methanogenic strains of bacteria screened for their ability to convert CO2 to CH4. High-pressure operation together with the presence of the bacteria enabled the breaking of kinetic barriers and reaching unprecedent levels of CO2 to CH4 productivity. CELBICON will therefore lead to radically new processes employing electrochemical- and bioreactors, which combine in a single process unit more functions to achieve higher conversion efficiencies with lower investment and operating costs. “Utilisation of CO2 by biological gas-converting bioprocesses is an innovative field with significant opportunities for environment, economy and society,” Fino concludes.