Single-port diabetes management
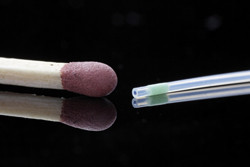
Many patients who have diabetes must take insulin to help regulate their blood glucose levels. Existing devices for subcutaneous insulin delivery are not accurate enough, leading to periods of hypo- or hyperglycaemia. This can cause both short-term and long-term complications that decrease quality of life and increase the cost of health care. EU-funded scientists are developing a solution to this problem with work on the project 'Single-port insulin infusion for improved diabetes management' (SPIDIMAN)(opens in new window). The SPIDIMAN system exploits a novel glucose-sensitive coating with a fluorescent dye (the glucose sensor) that can be applied onto standard insulin infusion sets. It is integrated into a single-port artificial pancreas system, an important simplification compared to the two needles typically required to measure glucose concentrations and to infuse insulin. This glucose sensitive layer is coated on the insulin infusion needle. Depending on the glucose level in the tissue it will emit light through the skin. A small optical reader is patched on the skin and interprets the emitted fluorescent light signal to glucose concentrations and will calculate the required insulin dose. Scientists have designed the transdermal optical glucose sensor for use with steel cannulas for catheter insertion. Novel coating technology ensures adhesion for patient safety and optimal performance. The second-generation glucose optical reader is much smaller and lighter than the first. It exploits custom-made optical filters for enhanced glucose discrimination and functions even when the cannula is perpendicular to the skin surface. The SPIDIMAN system has been classified as an active invasive medical device for short-term use, Class IIb. The first biocompatibility tests were passed and the team is conducting ongoing risk analyses that will continue through to the end of the project. A first paediatric clinical study is underway and 10 children aged 6–12 years have already completed the trials. SPIDIMAN technology exploits existing insulin infusion sets to keep unit costs low. With enhanced glucose sensing, the device will also lower costs associated with inadequate glycaemic control. Hospital costs are on average five times higher for diabetic patients with complications compared to those with none. The simplified one-port system with better sensing will be a particular benefit for young people whose lifestyles and higher glucose/insulin sensitivity make it harder to ensure quality care and quality of life.