Eggshell waste breaks into wound healing
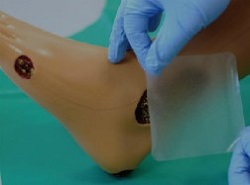
Delayed chronic wound healing is the so-called ‘vicious cycle’ of many illnesses. Chronic inflammation, excess protease, a key risk factor in healing, and tissue degradation are the hallmarks of this condition that can lead to further debilitation for the patient including a deterioration in psychological health. What’s more, already high healthcare costs are set to escalate the longer the wound goes unchecked. From eggshell waste to a healed wound with H2O2O funding A coveted Horizon 2020 SME instrument grant has allowed Norwegian company Biovotec AS and its partner Fitnesse Medical Ltd, Ireland to convert processed ESM to a new dissolvable wound dressing that can accelerate healing rates and slash costs. As Dr Henri-Pierre Suso, project coordinator and CTO of Biovotec points out: “H2020 funding has allowed Biovotec to formulate a new medical device product, upscale production and meet the cost per unit and perform all necessary pre-clinical tests as well as make a start with clinical trials.” ESM: Material mastermind behind wound healing ESM is a low-cost alternative to currently used collagen-derived dressings that are often too expensive to use. Based on a novel biomaterial derived from ESM, it is extracted from waste eggshells. Protecting the egg and its chick during development, ESM is a thin, structural protein-rich lining that performs similar key functions in wound healing to the extracellular membrane in skin. Membrane material from the egg production industry waste is purified and then ground into a powder before incorporating into the wound dressing. The product resembles a transparent blister plaster that is applied directly to the wound and covered with any secondary dressing, foams or bandages. As such, it allows for size flexibility. ESM kick-starts the healing process by virtue of distinct properties. Its properties help reduce tissue damage in a chronic inflammation scenario. Moreover, it binds matrix metalloproteases (MMPs) to reduce their presence in the wound. MMPs are a key risk factor in delayed wound healing. Highly significant, ESM promotes formation of new connective tissue and microscopic blood vessels during the process. Apply on any size, shape or type of wound The global market for ESM dressing is massive - around USD 3 billion globally and this number is expected to steadily increase. Presently, Biovotec’s primary focus, venous leg ulcers, affects 1 % of the population and 3 % among these are over 80 years of age. Significantly, due to population ageing and first world diseases such as obesity, the incidence of venous leg ulcers is likely to increase. The product appears to be suitable for any type of wound – including bed sores and diabetes-related ulcers. The dressing has been designed in collaboration with leading European doctors in the field to fulfil the stringent demands caused by varying practices. Consistency with all secondary dressing types, ease of application on both dry and wet wounds and the ability to fit onto any awkward moving shape such as an elbow by cutting were all considered as paramount criteria for end users. Cost of unhealed wounds is at the top of the list and BIOCURE believes this issue will set the Biovotec wound dressing apart from the competition. “Reducing the price per patient without compromising on standard of care is vital and we believe that this cost effectiveness will set the ESM film device apart,” emphasises Dr Suso. ”We aim to market the product at a price significantly lower than many of the biological wound treatments currently available on the EU market.” Next on the agenda With pre-clinical studies complete and successful, BIOCURE has set its sights on finalisation of all clinical studies and demonstration that the dressing is safe and effective. “We have now submitted our dossier to the Medicines and Healthcare products Regulatory Agency, which is a prerequisite to be able to test our medical device clinically. This will lead us to CE marking followed by commercialisation in 2019,” Dr Suso concludes.