Sophisticated technology sheds light on protein misfolding process
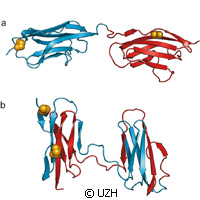
Protein folding is necessary for the function of three-dimensional structures in the body. However, some parts of functional proteins can remain unfolded, leading to the generation of various debilitating conditions like neurodegenerative diseases. Researchers in Switzerland and the United Kingdom have investigated protein misfolding using a sophisticated spectroscopic technique. Presented in the journal Nature, the study reveals how misfolding may compete with productive folding particularly in proteins containing many domains. The research was funded in part by a Marie Curie Intra-European Fellowship grant under the EU's Seventh Framework Programme (FP7). The University of Zurich and University of Cambridge researchers say misfolding occurs more frequently when the sequence of amino acids in the neighbouring protein domains is very similar. Experts call proteins the main molecular machines in our bodies. Their role is extensive; not only do they process nutrients and convert energy but proteins help cell structure and transmit signals in cells and the whole body. Proteins must adopt a well-defined, three-dimensional structure in order to ensure the performance of their specific functions, and for the most part they find this structure unaided once they have been formed out of their individual building blocks, amino acids, as a long chain molecule in the cell. But an error in the folding process results in proteins that are unable to carry out their functions. Thus, various disorders materialise, but avoiding the misfolding process is not as easy as one may think. The same molecular interactions that stabilise the correct structure of the individual proteins may trigger interactions between protein molecules, and in turn force them to misfold. Using a single-molecule fluorescence method, the team evaluated what happens when misfolding materialises. They probed domains, or sections, of titin, which is the body's largest protein. Titin contributes to the stability and elasticity of muscle fibres. It should be noted that individual titin domains can unfold while the muscle is heavily exerted to keep the muscle tissue from being damaged. But when the muscles relax again, it could lead to flawed folding of the domains. The team attached tiny dye molecules as probes in the protein for the purposes of their study. 'Using our laser-spectroscopic method we were able to determine distances on a molecular scale, i.e. down to a few millionths of a millimetre, through the energy transfer between the probes,' says one of the authors of the study, Professor Benjamin Schuler of the University of Zurich. Doing so allowed the team to successfully identify the structures correctly and to distinguish misfolded proteins. The researchers thus determined the proportion of misfolding. 'The study of different titin domains in our experiments revealed that the probability of misfolding increases if neighbouring domains are very similar in the sequence of their amino acids,' he explains. 'This seems to be a key evolutionary strategy to avoid protein misfolding and thus guarantee their maximum functionality.'For more information, please visit:Nature:http://www.nature.com/University of Zurich:http://www.uzh.ch/index_en.html
Countries
Switzerland, United Kingdom