EU research deciphers the maternal age effect
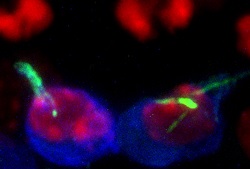
The age(opens in new window) at which women are giving birth in developed countries is rising and now many are choosing to have children in their mid-30s. But this is also the age when complications start arising. First, fertility decreases and once they have conceived, they can be faced with another major hurdle: from 10 to 25 % in their early 30s, women see the percentage of chromosomally abnormal haploid(opens in new window) eggs rise to over 50 % in their 40s. The ChromOocyte team aimed to provide a better understanding of the mechanisms behind this ‘maternal age effect’. Whilst this phenomenon was known to be caused by chromosome segregation errors in mammalian oocytes(opens in new window), several stones had yet to be unturned. “The few studies we could find had been performed on fixed cells and no data from live cell studies was available,” says Dr Melina Schuh, Director of the Max Planck Institute’s(opens in new window) Department of Meiosis and coordinator of ChromOocyte. “Moreover, systematic approaches to identify new genes that are specifically required for accurate progression through meiosis(opens in new window) in mammalian eggs were missing.” The team has made significant progress. They successfully conducted high-resolution live cell microscopy of human oocytes and imaged these cells throughout the different stages of meiosis. Besides, they developed a high-content phenotypic screening method for the systematic identification of mammalian meiotic genes. “Our approach has enabled us to deplete oocytes of several genes at once – for a total of 774 genes. From thereon, we analysed the function of these genes simultaneously by high-resolution imaging of chromosomes and microtubules in live oocytes. We scored each oocyte quantitatively for 50 phenotypes, which helped us generate a comprehensive resource of meiotic gene function,” Dr Schuh explains. The screening generated annotated data for meiotic progression in over 2 000 mammalian eggs. This allowed the team to systematically analyse which defects were linked to abnormal chromosome segregation during meiosis. The project identified multiple reasons for why errors are quite high in eggs from young and old women. In Dr Schuh’s words, “For example, human eggs often assemble a bipolar spindle by progressing through a prolonged multipolar spindle stage. This increases the likelihood of lagging chromosomes in anaphase. We found that this phenomenon may be facilitated by a large number of abnormal kinetochore-microtubule attachments.” The project’s data suggest that spindle instability and transient multipolarity contribute to the high frequency of chromosome segregation errors in human eggs. “In terms of ageing, we found that chromosomes are falling apart as women get older. This could be because a 40-year old woman has 40-year old eggs and chromosomes.” In what is perhaps the project’s most surprising outcome, the team found that actin filaments permeate the microtubule spindle in the eggs of several mammalian species, including humans. “This was unexpected. Actin filaments have so far been described as having a role in cell shape or cell migration, but they were generally thought to be dispensable for chromosome segregation. We found that actin drives accurate chromosome segregation, and thus is essential to prevent chromosome segregation errors in oocytes,” Dr Schuh enthuses. ChromOocyte has enabled significant progress towards understanding why chromosome segregation in human eggs is so error prone. But many aspects of meiosis in human cells are still not fully understood, and Dr Schuh intends to keep pushing the boundaries of research over the coming years.