Metal stents for coronary heart disease patients set to become a surgical museum piece
Usually made of metal mesh, heart stents help prevent the coronary artery from becoming blocked again. But they remain for ever in the artery causing dreaded long-term effect such as late stent thrombosis. Bioresorbable vascular scaffolds (BVSs) are poised to replace metal stents due to their excellent clinical outcomes. The EU-funded Bi-Stretch-4-Biomed(opens in new window) project has responded to the need for thinner and stronger BVSs: “Beyond keeping the vessel open during the first 6 months after surgery, the BVS leaves behind a healthy blood vessel after the scaffold is gone, being completely absorbed by the body approximately 2-3 years after surgery,” says joint project coordinator, Fulvia Villani.
Pros and cons of one proposed new material
Although poly(L-lactic acid), known as PLLA, has been clinically approved for the construction of BVSs, it presents problems for the surgeon. Not as strong and stiff as metals, the scaffold is three times thicker than the metal stents, making it more difficult for surgeons to move through arteries to reach the site of the lesion. “Thinner scaffolds that can be seen by X-ray imaging during surgery would facilitate adoption of the technology and benefit thousands of patients worldwide,” explains Villani. The Bi-Stretch-4-Biomed team investigated reinforcement of PLLA with tungsten disulphide (WS2) nanotubes to achieve both the strength and X-ray opacity needed.
Encouraging results for the new nanocomposite
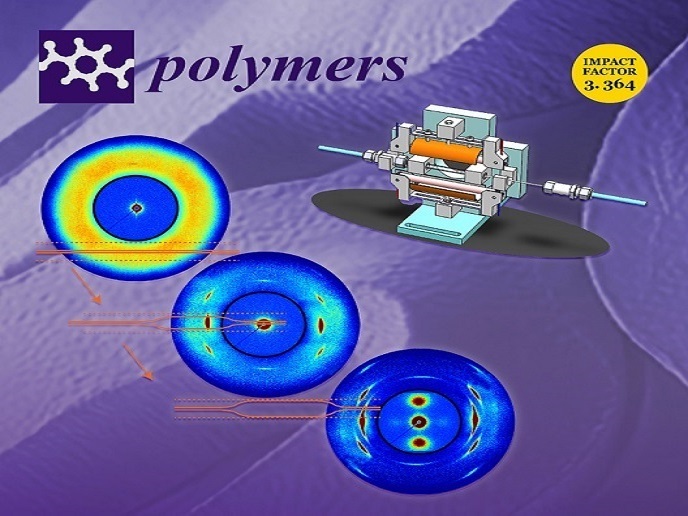
To develop the PLLA-WS2 nanocomposites fit for next-generation biomedical implants, researchers tested the material against strong deformation, as in tube expansion or biaxial stretching. They also provided materials’ parameters to model mechanical properties of PLLA-WS2 nanocomposites. In parallel, the investigators tested in vitro the biocompatibility of bare WS2 nanotubes and PLLA-WS2 nanocomposites as innovative material for new-generation, safe-to-implant BVSs. Results indicate that addition of WS2 reinforced the polymeric matrix of PLLA. Importantly, a relatively small amount of WS2 (0.1 % in weight with respect to PLLA) dramatically enhances oriented crystallisation, which can facilitate the production of BVSs with greater strength and a thinner profile. To monitor the morphological changes at a molecular level in PLLA-WS2 tubes (that will be then laser-cut into scaffolds), Bi-Stretch-4-Biomed developed an instrument specifically designed to impose a biaxial deformation to PLLA-WS2 tubes that reproduces the BVS manufacturing step of tube expansion(opens in new window). Experiments were held at a synchrotron light source in Argonne National Laboratory, United States (US). Cytotoxicity assays indicated that WS2 nanotubes (WSNTs) and PLLA-WS2 nanocomposites are well tolerated in vitro by selected human cells, a promising result towards the safety of the PLLA-WS2 scaffold.
Surprise element of people's commitment
Financial support for the project was supplied under the Marie Skłodowska-Curie Research and Innovation Staff Exchange programme (RISE)(opens in new window). As part of RISE, all partners had to pay visits to their counterparts' sites: “Full support was offered by each partner to compensate: from housing the visitors to the provision of necessary tools for developing joint research activities, a gesture that facilitated the exchanges,” emphasises Villani. “One real surprise wasn’t the technical implementation of the project but had to do with the real life of the people involved: they had to travel between the US and Europe for relatively long periods of time,” remarks Tiziana di Luccio, coordinator for the first 2 years of the project. Both coordinators are in full agreement that the consolidated networking became a launch pad for collaborations with academic and industrial companies mainly involved in biomed. The next step will be to direct the knowledge towards further investigation of in vivo testing of the new material and other steps of BVS manufacturing. Partners are monitoring new calls to fund future research.