A novel interaction mechanism in bone metastatic disease
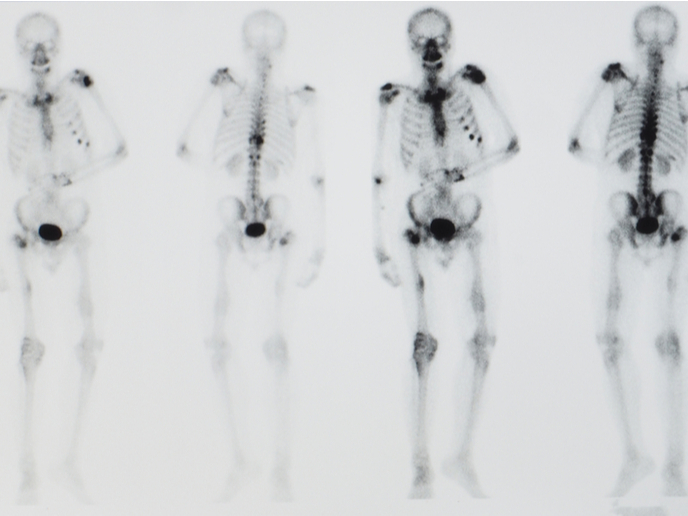
When cancer cells first invade bone tissues, they enter an environment largely regulated by osteocytes, which make up more than 90 % of our bone cells. Accumulating evidence indicates that they hijack the normal bone remodelling cycle, a homeostatic balance between bone formation and resorption. They can persist for years in a dormant state and become reactivated to form metastatic lesions.
An in vitro culture that recapitulates the in vivo interaction of cancer cells with bone
The scope of the META-DORM project was to shed light on the signalling mechanisms between metastatic cancer cells and the bone microenvironment. The research was undertaken with the support of the Marie Skłodowska-Curie programme, part-hosted in the lab of the late Professor Chris Jacobs at Columbia University in New York, and investigated the chemical and physical interactions between bone and cancer cells, during both dormancy and mechanical stimulation. “Our ultimate goal was to explore novel therapeutic targets to prevent or treat cancer metastasis,” explains the MSC research fellow Stefaan Verbruggen(opens in new window). Project scientists in the Knight Research Group at the School of Engineering and Materials Science at Queen Mary University of London established an in vitro co-culture of osteocyte cells together with different breast and prostate cancer cell lines, using micro-perforated membranes to allow them to exchange cytokine signals. “The idea was to develop an experimental strategy in the lab that mimics the in vivo signalling that takes place during cancer metastasis,” emphasises Verbruggen. Moreover, as mechanical loading of bone, driven by physical exercise, is critically important to healthy bone signalling in vivo, scientists also applied loading to the osteocytes to determine the effect on cancer cell invasion, migration and proliferation. This novel set-up represented an attempt to replicate signalling in vivo, allowing the investigation of the reciprocal crosstalk between native and metastatic cells under different conditions.
Research significance for future exploitation
The study’s main findings showed that osteocytes can prevent the proliferation of cancer cells and drive them towards more migratory behaviour. This suppression of tumour growth appears to be dependent on the presence of the osteocyte primary cilium(opens in new window), a slender organelle that protrudes from the cell’s surface and regulates the response of bone cells to mechanical forces and certain biochemical stimuli. However, cancer cells secrete the TGF-beta(opens in new window) cytokine that reduces expression of primary cilia in osteocytes. As a result, osteocytes can no longer inhibit cancer cell proliferation, leading to cancer lesions in the bone. “Our results indicate the presence of a feedback loop where cancer cells control their own metastatic growth by interfering with the bone’s possible endogenous anticancer mechanism,” outlines Verbruggen. The META-DORM study has shed light on a novel mechanism controlling cancer metastasis in bone, and a potential therapeutic strategy involving manipulation of primary cilia. Further exploration and exploitation of this interesting mechanism will have important impacts on European society, by increasing the knowledge base of the European scientific community in the fields of primary cilia biology and cancer research. The scientific team has received a multidisciplinary award from the United Kingdom’s Engineering and Physical Sciences Research Council(opens in new window) and Cancer Research UK(opens in new window) to develop a microfluidic organ-on-a-chip platform that better mimics the in vivo bone-tumour microenvironment. “This organ-on-a-chip model will allow us to test potential therapeutic strategies based on pharmaceutical modification of primary cilia,” concludes Martin Knight, Professor of Mechanobiology at the Institute of Bioengineering and Co-Director of the Centre for Predictive in vitro Models and the www.cpm.qmul.ac.uk/emulate/ (Queen Mary+Emulate Organs on Chips Centre) at Queen Mary University of London.