Precise metabolic mapping using MRI
Metabolic biomarkers serve as dynamic indicators of cellular activity, energy balance and the overall biochemical status of cells. In contrast to morphological biomarkers obtained through conventional imaging methods, these markers offer real-time insights into the functional state of organs and tissues. Monitoring metabolic biomarkers is of paramount importance in the field of medicine, as they are rapidly altered by therapies or the onset of diseases. This dynamic responsiveness provides a unique window of opportunity to assess the effectiveness of treatments, enabling clinicians to make informed decisions promptly, potentially sparing patients from ineffective or harmful interventions.
Utilising the power of high-field MRI
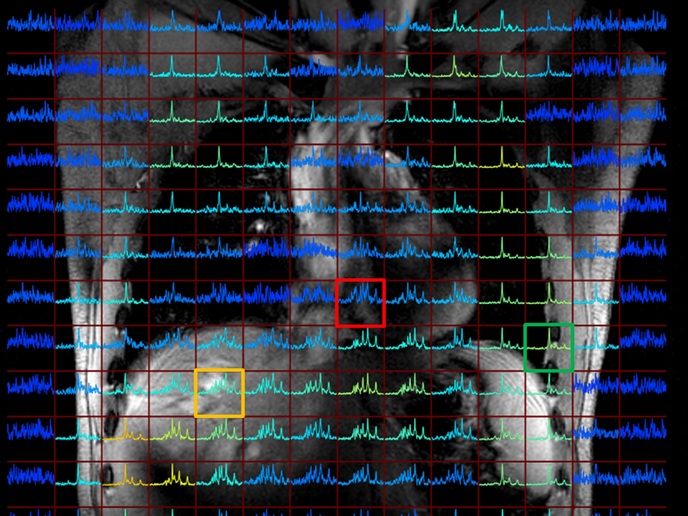
The EU-funded NICI project aims to harness the potential of emerging 7 Tesla magnetic resonance imaging(opens in new window) (MRI) scanners to provide profound insights into metabolomics throughout the entire human body. Conventional MRI scanners operate at 1.5 Tesla or 3 Tesla, but advancements in technology have led to the development of higher field strengths, such as 7 Tesla. The increased magnetic field strength in these systems offers several advantages, including high resolution and enhanced functional imaging. Compared to PET-CT(opens in new window), which is most often used to monitor glucose uptake in the body, metabolic MRI holds a distinct advantage. It allows simultaneous detection of multiple metabolite levels without the need for radioactive tracers, offering a safer and more comprehensive approach to imaging.
Mapping metabolic activity in the human body
Key findings from the project include the successful incorporation of radio frequency hardware into existing 7 Tesla MRI systems, facilitating metabolic MRIs without additional space requirements. This component is responsible for transmitting and receiving radiofrequency signals during the imaging process. Importantly, this integration allows for the implementation of metabolic MRI without requiring additional space, showcasing the adaptability of the technology. This advancement enabled the imaging of phosphorus (31P)-containing metabolites such as phosphomonoesters and phosphodiesters in large organs like the liver(opens in new window). This breakthrough offers the possibility to monitor the progression of liver diseases and responses to therapy. Patient feasibility studies also confirmed the detection of substantial metabolic alterations in lung carcinoma(opens in new window) after palliative chemotherapy and/or radiotherapy. “Mapping the metabolism throughout the human body with high accuracy and reproducibility is the most significant achievement of the NICI project,” highlights project coordinator Dennis Klomp.
Future directions
Looking ahead, the NICI project aims to further validate its technology and approach in other organs, targeting areas beyond hepatic metastatic lesions. The versatility of the NICI approach holds promise for identifying metabolic signatures applicable in cardiology, orthopaedics, paediatrics, and radiology. Plans are underway to provide software for metabolic imaging across all platforms, emphasising the project's commitment to creating a collaborative community by gathering open-source tools. In conclusion, the NICI project emerges as a transformative innovation in medical imaging, with the promise to reshape diagnostics, enhance patient care, and deepen our understanding of the intricate chemical processes within the human body.