Better fuel cell materials for cars
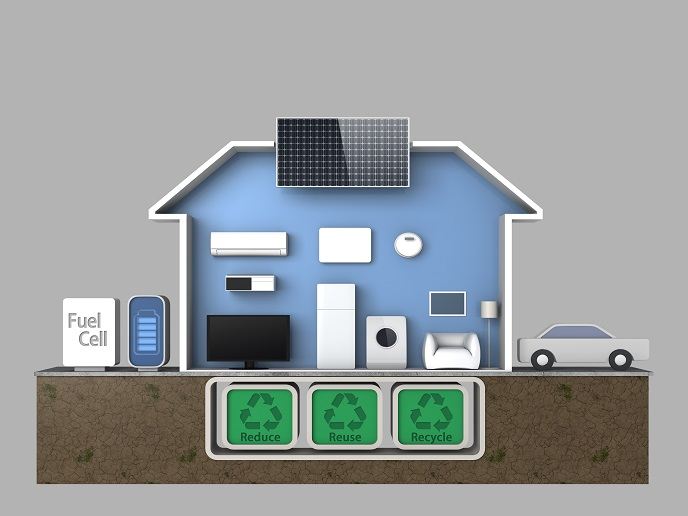
Fuel cells and hydrogen technologies are poised to be significant players in the game to reduce greenhouse gas emissions, now widely accepted to affect global climate change. Current fuel cell materials and technologies face temperature-dependent performance limitations. They cannot perform well at the desired automotive operating temperature of 120 degrees Celsius. Scientists initiated the EU-funded project 'Quasi-anhydrous and dry membranes for next generation fuel cells' (QUASIDRY)(opens in new window) to meet the challenge. The consortium focused on novel phosphonic acid-functionalised polymer membranes whose proton conductivity increases rather than decreases with temperature. Major development effort went into maximising conductivity of the new fuel cell electrolyte membranes. Researchers discovered that membranes functionalised with mixed proton-conducting materials, namely sulfonic–phosphonic or phosphoric–phosphonic groups, had improved properties compared to those observed for groups in isolation. Target conductivities were achieved with two membrane classes, and the novel approaches have made an important contribution to the field. Fuel cell electrodes consist of the catalysts, speeding the chemical reactions required to produce electricity, and so-called catalyst supports. QUASIDRY successfully developed both novel catalysts with improved activity and/or electrochemical stability at high-temperature operation, as well as supports with enhanced corrosion resistance and large surface area. Overall, scientists delivered four novel membrane classes, three new catalysts and a concept to improve polymer blending compatibility. These were studied under a range of conditions and cell architectures, with several showing significant improvements relative to benchmark materials. Further development of their potential could make QUASIDRY's novel polymeric membranes world-class fuel cell technology for transportation applications.