Möbius compounds from computational chemistry
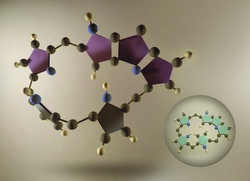
Möbius aromaticity is a property of cyclic compounds whereby a planar structure gains a half-twist. Such molecules with only one π surface and one edge offer exciting applications as optical materials, but are unpredictable and difficult to synthetise. The 'Designing viable Möbius aromatic systems using computational chemistry' (CCMOBIUS) project aimed to design novel Möbius aromatic compounds and fine-tune their electronic, magnetic and optical properties. The project focused on a class of molecules called expanded porphyrins, which are the higher homologues of naturally-occurring porphyrins. CCMOBIUS used a computer simulation method called density functional theory to understand the properties of various expanded porphyrins in the Möbius conformation. The project looked at the conformational preferences, aromaticity and dynamical switch between Hückel and Möbius conformations in pentaphyrins, hexaphyrins and heptaphyrins with a varying number of pyrrole rings. The project determined how overall macrocycle strain, aromaticity and metalation, as well as other physico-chemical properties influence Möbius compound stability. From this study, researchers developed a set of descriptors very useful for identifying porphyrinoids with an optimum balance between the ring strain imposed by the twist and the energy stabilisation due to Möbius aromaticity. Significantly, the optimum conditions for Möbius aromatic compounds based on penta-, hexa- and heptaphyrins were identified. The conformation of expanded porphyrins was found to be strongly dependent on the oxidation state, substituents and pH. In particular, they found that the addition of Group 10 metals locked the Möbius confomation in hexaphyrins. Very important, they identified viable Hückel-Möbius topological switches involving a drastic change in the nonlinear optical properties of the macrocycle. The computational results of CCMOBIUS are in excellent agreement with the experimental data available for these expanded porphyrins. The Hückel-Möbius topological switches developed in this project can be used as light-sensitive molecular switches for nanoelectronic applications.