Metalloenzymes in the nervous system
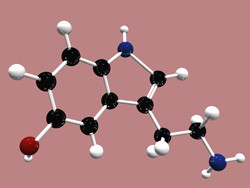
One third of the known enzyme catalysis reactions use metal, either as a cofactor, or incorporated into the enzyme molecule. Metals, such as iron, copper, zinc and magnesium participate in electronic transfer and empower numerous essential reactions. The EU-funded Marie Curie fellowship ICSTH (An integrated computational and spectroscopic investigation of the enzyme mechanism of tryptophan hydroxylase) was dedicated to metalloenzymes. In the course of the project, the initial focus shifted from tryptophan hydroxylase, an enzyme involved in the synthesis of serotonin, to binuclear copper enzymes. Peptidylglycine α-hydroxylating monooxygenase (PAM) and dopamine β-monooxygenase (DβM) catalyse hydroxylation of substrates crucial for functioning of the nervous system. Catalysis in both PAM and DβM is initiated by copper reduction and oxygen activation, but the mechanism of the reactions remains unknown. The project aimed to understand the mechanisms of oxygen activation, hydrogen abstraction, water binding and direct hydroxylation reactions catalysed by PAM and DβM. The complexity of copper catalytic centres required using a variety of the state-of-the art computational chemistry and spectroscopy methods. Defining the electronic structure of the initial enzymatic complex included determining the oxidation state of copper and the chemical nature of the oxygen adduct. The performed analysis of the hydroxylation mechanism explored the reaction paths, refined the transition states and possible reaction intermediates of each step. Combined quantum mechanical, molecular mechanical and molecular dynamics simulations helped study the effects of multiple mutations. This in turn allowed the exploration of the conformational effects on the reaction mechanism. The results of ICSTH enhanced our understanding of the crucial steps of hydroxylation mechanism of non-coupled di-copper containing enzymes. Such understanding is needed for advanced applications in drug design, protein engineering and biotechnology.