The molecular aetiology of inflammatory bowel disease
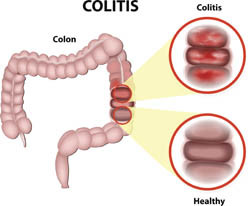
IBDs such as Crohn's disease (CD) and ulcerative colitis (UC) are believed to emerge from inappropriate inflammatory responses to normal components of the intestinal microbiota. IBD patients present with altered microbiota composition and especially adherent-invasive Escherichia coli (AIEC). Previous work by the EU-funded 'Role of microRNAs in host responses to Crohn's disease-associated adherent-invasive Escherichia coli' (MIRNA-AIEC) consortium showed that AIEC can invade human intestinal epithelial cells and macrophages, and survive. In addition, AIEC induces pro-inflammatory cytokine production and in mouse models they exacerbate intestinal inflammation. AIEC control is achieved through a functional mechanism of autophagy, a term used to describe the catabolic processing of dysfunctional cellular components by the cell itself. However, since AIEC prevalence is high in IBD, MIRNA-AIEC scientists presumed it could be due to dysfunctional autophagy. For this purpose, and based on earlier observations, they sought to identify the factors and mechanisms underlying the control of intracellular AIEC by autophagy. Given that recent evidence shows deregulation of miRNAs in CD patients, project scientists set out to determine the link between miRNAs and IBD susceptibility. The main objectives were to identify which miRNAs become altered upon endothelial cell infection with AIEC and how they induce cellular responses. The consortium identified 12 miRNAs that were altered in response to AIEC, and interestingly they targeted autophagy-associated genes. Inhibition of these miRNAs restored functional autophagy, and resulted in AIEC clearance as well as reduced inflammation. Taken together, the results of the study underscore the role of AIEC in autophagy response through altered miRNA expression. They also illustrate that miRNAs are promising therapeutic targets to inhibit the intracellular replication of CD-associated pathogenic bacteria. This information serves as a basis for the future investigation and discovery of new biomarkers and potential therapeutic targets for IBDs.