Nano-carriers against atherosclerotic plaques
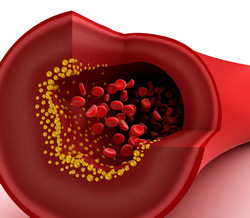
Aside from chronic blood flow problems, atherosclerosis can cause very serious acute problems. A piece of the atherosclerotic plaque can break off and lodge in a smaller vessel or a blood clot (thrombus) can form on the plaque's surface. If either of these completely blocks blood flow in a vessel in the brain or heart, it causes a stroke or a heart attack, respectively. Advances in nanotechnology and biomedicine now put targeted imaging, diagnosis and treatment within reach, but there are currently no nanoparticle-based systems approved for clinical use in cardiovascular diseases. A powerful consortium of 16 partners from 10 countries plans to change that with EU support of the project NANOATHERO(opens in new window) (Nanomedicine for target-specific imaging and treatment of atherosclerosis: development and initial clinical feasibility). The project is testing and exploiting different nanosystems in pre-clinical tests as well as in phase I clinical trials to establish clinical proof-of-concept for targeted imaging and therapy of atherosclerotic diseases in humans. The nanosystems consist of a nanoparticle carrier, coating or targeting materials and the imaging agent or drug inside the nanoparticle carrier. Researchers already selected nanoparticle carriers and three ligands to target atherosclerotic plaques. Magnetic resonance imaging and single-photon emission computed tomography imaging modalities will be exploited. The drugs that will be loaded inside the nanosystems to treat plaques and thrombus are drugs that have already been approved or are currently in clinical trials. The different Nanosystems are now in various phases of pre-clinical validation, including biocompatibility assessments and evaluation of imaging and therapy in vitro, ex vivo and in vivo. Some nanosystems are also in the in vitro and in vivo toxicity testing phase for which established and new animal models are being used. The first nanosystem has now been used in a clinical trial with atherosclerotic patients. The resulting data demonstrate a favourable pharmacokinetic profile and successful targeted delivery of an anti-inflammatory into plaque cells. Although this particular nanosystem e.g. the chosen drug inside the nanocarrier did not affect arterial wall inflammation as expected, this clinical trial delivers in itself already a promising proof-of concept for the targeting of a drug via a nanosystem in atherosclerosis patients. The results will be assessed thoroughly and exploited for the validation of upcoming combinations. NANOATHERO expects to deliver several key elements: the evaluation and testing of several different nanosystems for diagnostic and therapeutical use, the preparation of dossiers on regulatory issues, risk and ethical assessments, the preclinical evaluation of diagnostic and therapeutic systems as well as clinical investigations of patients at high cardiovascular risk.. Eventual application will have major impact on the millions with cardiovascular disease and will also open doors to similar therapies for a host of other devastating diseases.