Regenerative medicine for neurodegeneration
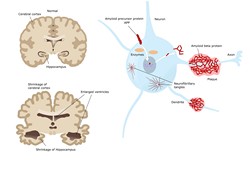
Neurodegenerative disorders such as Alzheimer’s disease as well as other neurological conditions have a devastating impact on the quality of life of sufferers and their families. Although brain function deterioration is a natural cause of ageing, neurodegeneration induces an accelerated neuronal cell dysfunction. Considering the lack of ability of the CNS to self-regenerate, interventions that trigger damaged neurons to regenerate are promising. In this context, the scope of the EU-funded NEUROFGL (Development of a novel FGL therapy and translational tests for regenerative treatment of neurological disorders) project was to develop a novel approach based on the use of modulators of the fibroblast growth factor (FGF) receptor. The allosteric compounds, referred to as FGL, mimic the activity of the surface neural cell adhesion molecule (NCAM). Emerging evidence has demonstrated a positive disease-modifying effect of FGL in various in vivo models of neurodegeneration. A phase I clinical study had also proven that FGL peptides are well tolerated and safe. The ultimate goal of NEUROFGL was to reach clinical proof-of-concept for this promising and novel regenerative therapy. To achieve this and provide a more robust basis for the implementation of FGL, researchers studied its mechanisms of action. Histological evaluation of the hippocampus of treated animals demonstrated increased plasticity and neuronal stem cell proliferation and differentiation towards an oligodendrocytic fate. In naive rats, FGL caused the expansion of the subventricular zone, one of the main endogenous stem cell niches of the brain. The next step was to conduct pharmacological evaluation of the toxicity and stability of FGL in preclinical models. In a clinical context, the consortium performed a single ascending dose (SAD) study to validate the safety and tolerability of FGLs, illustrating a similar pharmacokinetic profile to animals. From a diagnostic perspective, the consortium set out to develop a novel PET tracer (bromodeoxyuridin analogue) for detecting neurogenesis in vivo. The rationale was to facilitate early clinical assessment, thereby maximising the chance that FGL and other regenerative therapies can benefit patients. Overall, the activities of NEUROFGL open new avenues for the development of other drugs with a similar mechanism of action as well as therapies for neurodegenerative disorders.