Automated stem cell production
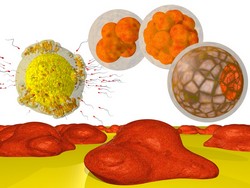
Cell-based screening is a widely used method in microbiology studies, including for determining drug effects and assessing the safety of new drugs. A recent improvement for drug discovery is human induced pluripotent stem cells (hiPSCs), which also opens up personalised and/or regenerative medicine. The EU-funded DROPTECH(opens in new window) (Hanging drop based automated and parallelized cell technology platform for production and testing) project developed automated handling processes for pluripotent stem cells, including hiPSCs. The new processes include integrated readout methods for use of the cells in high-throughput and high-content assays. The platform is designed to rapidly produce embryotoxicity screenings and reduce the need for animal tests. Work began with analysis of the system requirements, and researchers developing appropriate specifications. The team also developed various standard cell workflows. The workflows include maintenance, cryopreservation, recovery, differentiation of the hiPSCs into cardiac cells, and the shipment of quality-controlled hiPSC lines. The complete workflow was adapted to incorporate several refinements. Researchers developed a system for automated production and handling of stem cell aggregates in hanging drops. Such drops allow control of cell culture conditions in small volumes. The platform allows standardised high-throughput screening in applications, including lead compound identification, over-toxicity testing and cell preparation for regenerative medicine. The embryonic stem cell test (EST) is the only currently approved method for testing embryotoxicity in vitro. Hence, researchers used the test to validate the project’s platform. Project developments yielded automation of several EST processes using robotic and microfluidic systems. The processes include cell expansion, embryoid body formation, cardiac differentiation, compound addition in hanging drops and transfer to 2D conditions. The cell system used was also successfully transferred to hiPSC usage. DROPTECH’s platform includes monitoring solutions to automatically gather information about the development potential of whatever cell model is being used. Results showed no differences compared to the previous complex manual procedure. The resulting automated system allows fast and efficient handling of cell cultures in droplets, which will facilitate the assay process. The new method will aid drug discovery, toxicity testing and regenerative medicine.