The molecular events that determine the acquisition of developmental competence during oocyte maturation
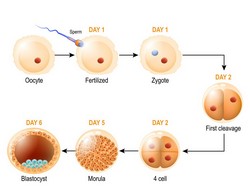
The final phase of oocyte differentiation, fertilisation and early embryo development occur in the absence of transcription. Therefore, cell cycle progression, chromosome segregation and genome reprogramming rely on translation of stored mRNAs, until the activation of the embryo genome. Subtle disturbances of translation allow the oocytes to undergo apparently normal maturation but, once fertilised, these oocytes fail to develop into an embryo. The EU-funded MATERNA project proposed that follicular cells surrounding the oocytes regulate the activation of translation of specific mRNAs in oocytes and investigated whether deregulation of this process is responsible for impaired embryo development, also during reproductive aging. To study the biochemical mechanisms by which somatic cells regulate the translational programme of mouse oocytes, researchers used loss and gain of function approaches, treating oocytes with hormones/growth factors or inhibitors of intracellular pathways. Project results underscored PI3K/AKT as a key pathway linking somatic cell activation to induction of oocyte-specific translation. By using a genetically engineered mouse model, where AKT was constitutively activated in the oocyte, scientists discovered that this pathway increased translation of specific mRNAs and embryo development. Furthermore, they observed that transcription in the cumulus cells is required to propagate AKT activation in the oocyte and that the AKT pathway is a common downstream following stimulation with EGF-like growth factors and FSH. Overall, project findings demonstrated that mammalian embryo development strongly depends on the fulfilment of the oocyte translational programme before ovulation. Disruptions in this programme contribute to decreased female fertility. Collectively, the results provide a molecular rationale for the use of EGF-like growth factors and FSH during oocyte in vitro maturation procedures to promote oocyte developmental competence. Finally, manipulation of the PI3K/AKT pathway may represent a new line of intervention to activate translation, therefore enhancing the quality of oocytes.