Medical imaging radiomics: beyond what meets the eye
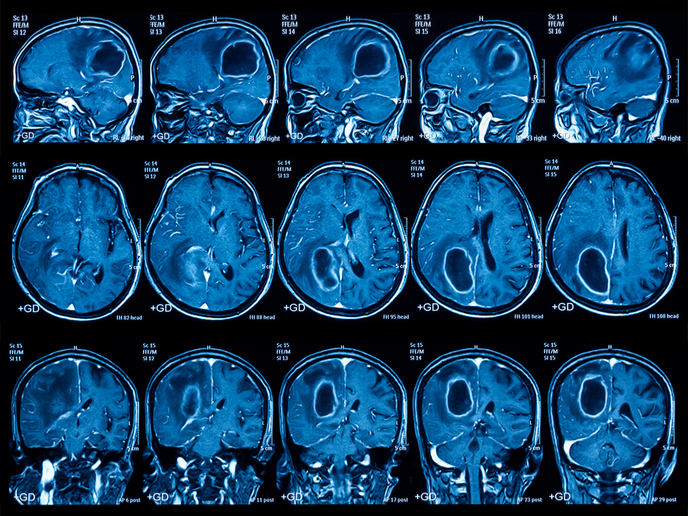
Recently, the emergence of advanced imaging analytics (radiomics) has transformed imaging science, providing deep quantitative features that offer spatial and longitudinal understanding of tumour biology. This technology utilises radiologic patterns for the characterisation of tumour phenotype over time and after treatment, and provides critical predictive information. Aligning radiomics with MRI Magnetic resonance imaging (MRI) is one of the most promising techniques for cancer detection and progression. However, variation in the magnetic field strength produces variable MRI images posing a challenge for radiomics analysis. Scientists of the EU-funded REACT project addressed this issue through an innovative solution, which extracts quantitative information from MRI medical images in a standardised way. This is achieved through a set of stable tumour phenotype features such as shape and texture (along with more features undetectable by the eye), which correlate with tissue pathology irrespective of magnetic field strength or vendor. “Our goal was to provide clinicians with more information and to assist them in treatment decisions,″ emphasises Dr Samir Barakat, the principal data scientist of PT Theragnostic B.V. a company dedicated to the development of tailored therapies for cancer patients. REACT researchers harnessed the power of advanced artificial intelligence such as machine deep learning to deliver accurate and robust clinical decision based on standard clinical imaging. Radiomics can be used as a surrogate endpoint to the traditional progression-free survival, and can provide important information on the effectiveness of specific drugs. Importantly, the standardised methodology generated during the REACT project makes analysis feasible across multiple centres and vendors, reducing variability. Furthermore, the data obtained enable patient stratification and facilitate the prompt detection of complications. The future of radiomics Tumour spatial and temporal heterogeneity alongside the plethora of available treatments pose a significant challenge to treatment decision. Identification of the biological differences between tumours through biomarkers is a promising approach towards personalised therapies. Ideally, this identification should be non-invasive and reflect the complexity of solid tumours. Radiomics is a powerful tool that can utilise routinely acquired images to portray a comprehensive view of tumour characteristics and guide the design of personalised treatments. Using the evolution of hundreds of image-derived features, it allows for more sensitive and robust identification of tumour types than currently possible. At the same time, radiomics can be used to monitor the development and progression of the disease or its response to therapy. In contrast to the expensive genomic and proteomic technologies, radiomics quantifiable data can be employed in routine clinical care. Through a platform for the high-throughput mining of quantitative image features, REACT aims to advance knowledge and improve the diagnostic, prognostic, and predictive accuracy of the existing clinical decision support system. Undoubtedly the future of cancer treatment lies in prompt diagnosis and personalised medicine. Providing the right treatment to patients in the long term will increase survival rates, improve the quality of life for patients and reduce healthcare costs. In view of the future, Dr Bakarat believes that “radiomics has the power to progress MRI beyond the state of the art in oncology.″