Sophisticated technology reveals mystery behind van Gogh's colours
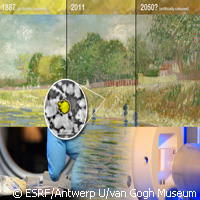
Vincent van Gogh's art is duller than what it used to be but no one has ever been able to figure out why - until now. An international research team has discovered that sunlight is potentially responsible for triggering a chemical reaction in the yellow chrome paint used by the Dutch post-Impressionist Master painter, turning it into a dull brown colour. The findings are presented in the journal Analytical Chemistry. Led by experts from Belgium, Italy and the Netherlands, the researchers used a super-sensitive microscopic X-ray from the European Synchrotron Radiation Facility (ESRF) in France and German Electron Synchrotron (DESY), as well as infrared radiation, electrons and X-rays at the Universities of Antwerp (Belgium) and Perugia (Italy) to reveal how the bright yellow colours of van Gogh's most famous paintings, as well as other artists of the 19th century, became lacklustre and faded over time. Their research suggests shielding affected paintings as much as possible from ultraviolet (UV) rays and sunlight. 'For every Italian, conservation of masterpieces has always mattered,' explains the lead author of the paper, Letizia Monico from the University of Perugia. 'I am pleased that science has now added a piece to a puzzle that is a big headache for so many museums,' adds Ms Monico, a PhD student and Italian chemist who for one year worked with Koen Janssens, the lead researcher of this study, at Antwerp University in Belgium. Their use of innovative tools helped the team piece together this fascinating mystery. Using an X-ray beam of microscopic dimensions, they revealed a complex chemical reaction taking place in the very thin layer where the paint meets the varnish. The experts say sunlight can penetrate just a few micrometres into the paint, but over this short distance, it will set off a previously unknown chemical reaction, turning chrome yellow into brown pigment. The result is that the original composition is changed. Vincent van Gogh (1853-1890) was way ahead of his time, choosing to use colours that conveyed mood and emotion, rather than using them realistically. Chrome yellow, for instance, allowed him to achieve the intensity of his series of Sunflowers paintings, according to the researchers. It has long been recognised that yellow chrome paint darkens under sunlight. However, not all paintings are affected and the speed at which they are affected varies. Artists in the mid-20th century found new alternatives since chrome yellow is toxic. But van Gogh was forced to work with what he had. Chrome yellow is good for preservation, and its popularity is rising again. So the team used a two-step approach to solve this puzzle. They initially compiled samples from 3 historic paint tubes, ageing them artificially for 500 hours using a UV lamp. They focused on one sample derived from a paint tube belonging to Flemish Fauvist Rik Wouters (1882-1913). The state-of-the-art analysis revealed the darkening of the top layer as connected to a loss of the chromium in the chrome yellow, from Cr(VI) to Cr(III). The team also reproduced Wouters' chrome yellow paint and discovered that UV light could trigger a darkening effect. Step two focused on using the same methods to assess samples derived from affected areas of van Gogh paintings: View of Arles with Irises (1888) and Bank of the Seine (1887), currently being displayed at the van Gogh Museum Amsterdam. Their investigation suggested that the reduction reaction from Chromium (Cr)(VI) to Cr(III) materialised in both van Gogh paintings. The microscopic X-ray beam also indicated that Cr(III) was particularly prominent in the presence of chemical compounds that contained barium and sulphur. The team believes that van Gogh's technique of blending white and yellow paint could be responsible for the darkening of his yellow paint. 'This type of cutting-edge research is crucial to advance our understanding of how paintings age and should be conserved for future generations,' says Ella Hendriks of the van Gogh Museum Amsterdam. In terms of what's in store for the researchers, Dr Janssens says: 'Our next experiments are already in the pipeline. Obviously, we want to understand which conditions favour the reduction of chromium, and whether there is any hope to revert pigments to the original state in paintings where it is already taking place.'For more information, please visit: European Synchrotron Radiation Facility (ESRF):http://www.esrf.eu/Analytical Chemistry: http://pubs.acs.org/journal/anchamVan(opens in new window) Gogh Museum Amsterdam: http://www.vangoghmuseum.nl/vgm/index.jsp(opens in new window)
Countries
Belgium, Italy, Netherlands