Revealing the role of glucose metabolism in colorectal cancer
In the 1950s, a German scientist called Otto Warburg(opens in new window) found that cancer cells metabolised glucose differently from normal cells. Today we know that most cellular metabolic pathways are ‘rewired’ in cancer cells, known as metabolic reprogramming. As this allows cancer cells to grow, proliferate and survive, it is intimately linked to tumour progression, metastasis and therapeutic resistance. Recent colorectal cancer(opens in new window) studies have suggested that glucose metabolism influences intestinal stem cells, from which this type of cancer originates. Yet the precise mechanism by which this happens has not been understood. MDCRC, a Marie Skłodowska-Curie Actions(opens in new window) project, identified and characterised a new intestinal epithelial cell type that is highly glycolytic – that is, has a high glucose uptake. This allows cancerous tumours to grow and spread. These glycolytic cells also had the stem cell-like ability to generate all the different cells present in the intestinal epithelium(opens in new window). They were also shown to contribute to the initiation of intestinal tumours. “Our findings could potentially inspire new therapies for the treatment of colorectal cancer, by interfering with the specific metabolic adaptations of tumour-initiating cells,” says Marie Skłodowska-Curie fellow Carlos Sebastián from the Candiolo Cancer Institute(opens in new window), the project host.
Discovery of highly glycolytic cells
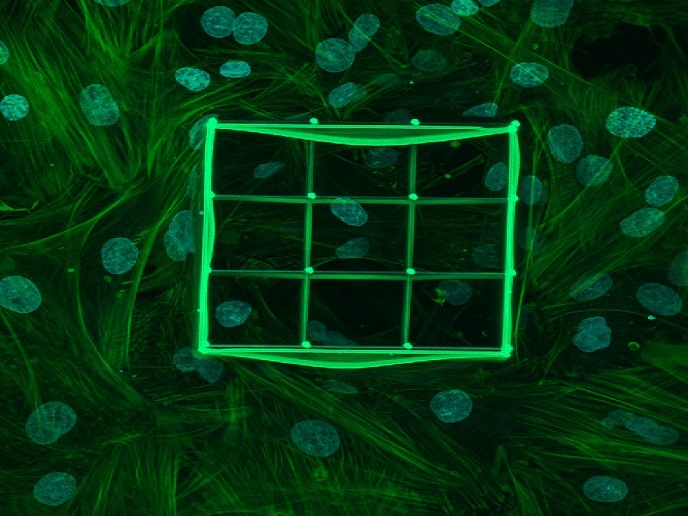
Previous work by the MDCRC team had demonstrated that the reprogramming of glucose metabolism was a critical step to initiate intestinal tumours. Concomitantly, other researchers had discovered that the transformation of intestinal stem cells gave rise to intestinal tumours. The MDCRC project combined the two observations to propose that glucose metabolism could be regulating tumour initiation by affecting the biology of intestinal stem cells. The team developed a novel, fluorescent, genetically encoded metabolic reporter to visualise, track and isolate cells with different metabolic properties within the intestinal epithelium. “This system enabled for the first time, the imaging, tracking and characterisation of cells with different metabolic properties within the native microenvironment of living tissue, providing an unprecedented level of detail,” explains Sebastián. The team used 3D structures called organoids – derived from mouse models of colorectal cancer – that mimicked the intestine’s cellular composition. “By studying the formation of these organoids, we discovered highly glycolytic cells, many with stem cell properties. Crucially, assays confirmed that these glycolytic cells were also engaged in tumour-initiating activity,” adds Sebastián. Immunostaining(opens in new window) and gene expression experiments enabled the team to characterise the phenotype of these cells, indicating which specific type of intestinal epithelial cell their highly glycolytic cells were. Using chemical and genetic techniques, the team also inhibited glycolysis in these cells which reduced their stem cell properties – they formed fewer organoids in assays. As only stem cells can give rise to these organoids, this demonstrated that glucose metabolism regulates stem cell activity.
Towards new therapies
Colorectal cancer is the second most common European cancer and the third worldwide. Although recent advances in the molecular genetics of colorectal cancer have helped design new targeted therapies, most patients still relapse and succumb to this deadly disease. As well as the obvious impact on sufferers and their families, this places a significant burden on health systems. “By increasing understanding about the underlying molecular mechanisms, our results could benefit patients,” concludes Sebastián. The team are currently continuing with a series of experiments to fully characterise the range of metabolic activity associated with colorectal cancer. They are also already applying their findings to humans using organoids derived from patients’ tumours, which are transplanted into mouse intestines to follow tumour growth.