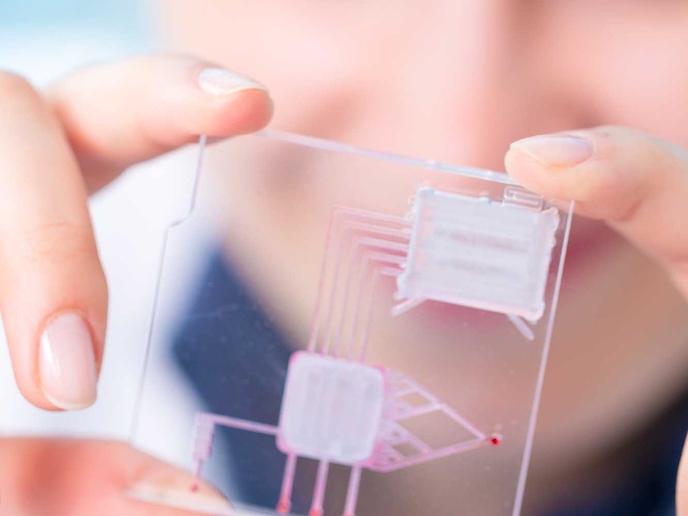
Hybrid manufacturing of microfluidics devices benefits medical diagnostics
Microfluidic(opens in new window) microelectromechanical systems (MEMS) are nanosized precision devices which combine mechanical and electrical components that are capable of fluid manipulation. Able to handle sample volumes of less than a millilitre, they can be used as medical diagnostic devices with intelligent functions such as molecular detection and signal analysis. Dubbed ‘labs-on-a-chip’, these devices, often made from polymers(opens in new window) for their advantageous properties, offer low reagent volumes, rapid processing and precise control, but materials and manufacturing make their production challenging. “The functional and commercial feasibility of polymeric microfluidic devices depends on the cost-effective integration of multiple functionalities, materials and processes with high precision and repeatability,” says Costas Charitidis, coordinator of the EU-funded M3DLoC(opens in new window) project and director of the R-Nano lab at the National Technical University of Athens(opens in new window) (NTUA), the project host. M3DLoC has developed a prototype digital manufacturing pilot line capable of producing just such polymeric microfluidics, with bespoke solutions from low-volume runs to pilot-scale batches of 10-1 000 devices, depending on size, assay requirements and overall complexity of feature design. “By using 3D printing technologies in hybrid additive-subtractive process chains, we established a fully digital and automated production line. This unique, internationally leading pilot production line, could open up new market opportunities in the biomedical industry,” adds Eleni Gkartzou from the project’s NTUA coordination team.
The hybrid manufacturing process
M3DLoC’s pilot production line consists of five processing stations and two in-line metrology systems for component quality assurance. The steps in the manufacturing sequence can be altered, combining extrusion-based additive manufacturing(opens in new window) (AM) and inkjet printing, with micromachining and laser processing. “This integrated approach links flexible microfabrication and multimaterial integration to manufacture high aspect ratio microfluidic features on polymer substrates, while incorporating microstructured carbon-based electrodes and precisely deposited biomolecules, down to picolitre volumes,” explains Dimitrios Fantanas, also from the NTUA project coordination team.
Integration of sample analysis within a cartridge
The project demonstrated the capability of the production line to produce devices suitable for clinical diagnostics, using protocols and assays developed by microfluidic experts and end users, for viral (HIV, SARS-CoV-2), bacterial (drug-resistant tuberculosis bacterial strains) and cancer (epidermal growth factor receptor mutations) biomarkers. Various parameters – fluid control, sensor designs and manufacturing – were simulated against end-user requirements. Additionally, bioderived thermoplastic materials and water-based conductive inks with low environmental impact were developed, capable of replacing more traditional materials such as silicon and glass. “The results demonstrated that M3DLoC devices are suitable for the analysis of challenging clinical samples and are comparable to gold-standard clinically validated methods,” notes Charitidis.
A complete value chain for multiple applications
The M3DLoC project and its partners successfully dealt with the restrictions of the COVID-19 pandemic, when the M3DLoC AM system was used to produce personal protective equipment for healthcare workers. They surmounted pandemic hurdles to develop a complete value chain from prototype design, material development, production and implementation, to performance assessment. “Inexpensive, high-performance assays offer earlier diagnosis, personalised medicine and better patient follow-up, reducing healthcare costs and improving quality of life,” says Charitidis. Such diagnostic devices could also improve quality control techniques across the chemical, oil and gas, pharmaceutical and food industries, as well contributing to systems for environmental and agricultural monitoring. “The pilot line is available for end users requiring a microfabrication facility in an open access industrial setting for pilot production of medical devices. It accelerates the transition from research and development to pre-commercial production, through rapid prototyping, using fully digitised manufacturing,” concludes Charitidis.