Point-of-care tests for syphilis and HIV
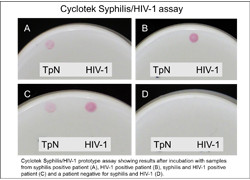
Mother to child transmission of syphilis and HIV are major causes of severe morbidity and early death of infants. To reduce the risk of congenital transmission the mother must be treated, which necessitates the availability of an accurate diagnostic test for onsite screening of pregnant women. Scientists on the EU-funded PREVENTIT(opens in new window) (Assured point-of-care device for syphilis and HIV in pregnant women and new born) project set out to develop tests for the point-of-care screening of HIV and syphilis infections in pregnant women. For this purpose, the consortium explored two different platforms, namely the lateral flow assay (LFA) and the Cyclotek assay specifically designed for multi-analyte testing. The assays were based on HIV and syphilis capture and colloidal gold-labelled detection probes associating with antigens present in the serum. Scientists had to include reagents for the detection of antibodies against different HIV types. With respect to syphilis, the assay had to detect Treponemal antibodies, specific for T. pallidum antigens, and non-Treponemal anti-phospholipid antibodies, that recognised material released from damaged host cells. The LFA assay is limited by the number of probes that can be combined, and by the time the antigen is allowed to react with the immobilised capture probe. This format potentially limits sensitivity compared to the Cyclotek approach. Nonetheless, successive HIV/Syphilis LFA prototypes were developed that showed sufficient diagnostic performance. Cyclotek allows the immobilisation of multiple capture probes at defined positions, thereby permitting multiple rounds of interaction and thus higher sensitivity. Scientists investigated the possibility of multiplexing HIV and syphilis detection, but the differential nature of the antigens resulted in suboptimal assay performance. Automated reading of this assay helped minimise error and improve sensitivity. Although the diagnostic accuracy of the HIV1,2/Syphilis LFA might require further fine-tuning prior to clinical application, the overall concept of point-of-care testing proved feasible. The lack of access to healthcare laboratories and limited awareness of many of the neglected poverty related diseases in developing countries facilitates the spread of diseases such as HIV. The introduction of point-of-care tests such as the ones developed by the PREVENTIT team should help mitigate this situation.