A novel epigenetic test for lung cancer detection
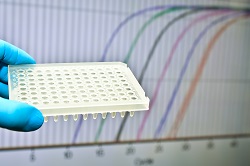
Lung cancer diagnosis usually takes place through the appearance of suspicious symptoms or accidentally by clinical imaging performed for other indications. Apart from symptoms and radiological findings in the lung, prognosis also depends on patient smoking history and age. Recently, the National Lung Screening Trial in the US demonstrated a 20 % mortality reduction in patients with an elevated risk for lung cancer using low-dose computed tomography (LDCT). However, LDCT is associated with a high false positive rate, hampering its widespread use. In addition, clinical evaluation of patients through a CT scan and a bronchoscopy is not always possible as the lung cancer tissue may be inaccessible or the cytological specimen unsuitable for assessment. To overcome limitations in lung cancer screening, scientists of the EU-funded proLungPlasma(opens in new window) project proposed an alternative approach based on epigenetic biomarkers – markers of heritable changes in gene function that do not involve alterations in DNA sequence. Methylation of DNA is an important epigenetic process involved in fundamental biological processes such as development and cell differentiation. “Our approach exploits the fact that aberrant DNA methylation plays a major role in cancer development,″ explains project coordinator Dr Gunter Weiss. An epigenetics test for lung cancer detection The proLungPlasma business innovation project was designed to validate an epigenetic biomarker test for lung cancer detection in plasma samples. The work was based on previous findings demonstrating that the methylation of SHOX2 could be used for the identification of lung cancer in bronchial lavage with very high accuracy. “We aim to deliver a blood-based molecular diagnostic instrument for complementing systematic lung cancer screening that reduces false positive rates,″ continues Dr Weiss. The test comprises a real-time PCR assay for the detection of SHOX2 and PTGER4 gene methylation in DNA isolated from patient plasma. SHOX2 and PTGER4 gene methylation can be detected by specific amplification of circulating DNA present in the bloodstream. Emerging evidence indicates that measurement of SHOX2 and PTGER4 methylation in plasma DNA has aided in detection of lung cancer. An added bonus, they can be used for differentiating between malignant and non-malignant forms of lung disease. Following reagent optimisation and product development, the next step was validation of the Epi proLung test in a clinical performance study. In a study with over 350 patient samples, the test showed significant discriminatory performance in distinguishing lung cancer with area under the curve of 0.83. Sensitivity was 85 % at a 50 % specificity level. Considerable effort went also towards the manufacturing process, scaling up production to meet technical requirements as well as economic needs. The future in lung cancer detection The Epigenomics SME has very successfully engaged in epigenetic research from 1998, resulting in an outstanding proprietary platform for epigenetic biomarker discovery for cancer. The Epi proLung test comes as an addition to the company’s outstanding portfolio of proven biomarkers designed for the detection of cancers. Overall, the project demonstrated that assessment of DNA methylation markers in blood plasma provides a reliable diagnostic method for lung cancer. Dr Weiss predicts that “the Epi proLung test may serve as a suitable tool to aid in the diagnosis of lung cancer in patients at increased risk for the disease.″ Successful implementation of the test would be expected to lower false positive rates, reduce unnecessary procedures and associated psychological burdens, and most importantly, result in detection at earlier stages with better prognosis.