Putting a cap on influenza
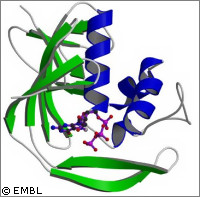
European scientists have shed new light on how the influenza virus hijacks the cell production machinery of its host. Their findings could lead to the development of new drugs to combat influenza pandemics in the future. The work was supported by the EU-funded FLUPOL project, which is financed by the EU to the tune of €1.97 million. One of the greatest fears among governments and health agencies is of a devastating flu pandemic. They are worried that highly virulent strains of bird flu, like H5N1, may develop the ability to spread from human to human. As a result, new treatments and methods are being sought to halt the spread of the virus. In this latest study, structural biologists led by Stephen Cusack of the European Molecular Biology Laboratory (EMBL) scrutinised one of the ways in which the influenza virus takes over the workings of infected cells. Their results are published in the journal Nature Structural and Molecular Biology. The researchers obtained a high-resolution image of a key protein domain, whose function is to enable the virus to multiply by hijacking the host cell's protein production machinery. When the influenza virus infects a cell, it begins to multiply. Critical to this process is a protein called viral polymerase. This enzyme copies the genetic material of the virus and helps to produce more viruses. A component of the polymerase, known as PB2, plays a vital role in stealing an important 'tag' from the host cell's RNA molecules. It then uses this tag to direct the machinery of protein production towards the synthesis of viral proteins. Scientists belonging to the research teams of Stephan Cusack and Darren Hart at EMBL Grenoble have discovered the PB2 domain responsible for binding the tag. From this they have produced crystals which have been studied using the powerful X-ray beam of the European Synchrotron Radiation Facility (ESRF). 'Viruses are masters of cunning when it comes to hijacking the normal functioning of the host cell. The influenza virus steals a password from host messenger RNAs, molecules that carry the instructions for protein production, and uses it to gain access to the cell's protein-making machinery for its own purposes,' says Dr Cusack. In reality the password takes the form of a short extra piece of RNA known as a 'cap'. This cap must appear at the beginning of all messenger RNAs (mRNAs) in order to direct the cell's protein-synthesis mechanism. The viral polymerase binds to the host cell mRNA via its cap, removes the cap and adds it to the beginning of its own mRNA. This process is described as 'cap snatching'. The hosts cell's protein production mechanism is then able to recognise the capped viral mRNA, allowing viral proteins to be produced at the expense of host cell proteins. The EMBL researchers produced an image showing a PB2 domain bound to a cap, revealing for the first time the individual amino acids involved in recognising this particular structure. Scientists were able to identify the cap sandwiched between two PB2 amino acids. Although this recognition mechanism is not unlike other cap-binding proteins, its structural details are distinct. Colleagues in Madrid based at the Centro Nacional de Biotecnologia demonstrated that disruption of the PB2 cap-binding site stops the influenza virus from replicating. 'These findings suggest that the PB2 cap-binding site is a very promising target for anti-influenza drugs,' says Darren Hart. 'Our new structural insights will help us design mimics of the cap that would inhibit viral replication and hence reduce the spread of virus and the severity of the infection.'