How extinct marine lizard reveals endogenous proteins from within
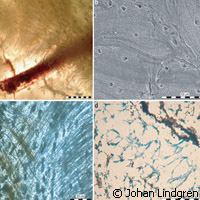
A team of scientists in Sweden has found a fossil of a giant, extinct marine lizard (mosasaur) dating back to the Late Cretaceous period, around 100 to 65 million years ago. But what is unique about this research is that they succeeded in finding authentic remains of an animal that no longer walks on this earth ... embedded in stone. Presented in the journal Public library of Science (PLoS) ONE, the results provide insight into how the recovered biomolecules are primary and not contaminants from bacterial biofilms or collagen-like protein. Past studies focused on identifying collagen-derived peptides in dinosaur fossils based on various methods including tandem mass spectrometric analyses of whole bone extracts. The downside of using this type of method is that location-specific tissues could not be used, thus forcing researchers to depend on whole bone extracts instead. Another drawback is that it is unclear as to whether amino acid sequences obtained from such an analysis are genuine. For their part, the Lund University researchers used sophisticated technology to link proteinaceous molecules to bone matrix fibres isolated from the fossil aged 70 million years, specifically a humerus (IRSNB 1624). Their diligence paid off. Using synchrotron radiation-based infrared microspectroscopy at the MAX IV Laboratory at Lund, the team showed that amino acid-containing matter remains in fibrous tissues obtained from the mosasaur bone. That is, the analyses provide solid evidence to suggest that primary organic molecules, such as collagen, or its degradation products, are preserved in the fibrous bone tissues of the humerus in question. 'This technique provides information on complex organic molecules in selected microstructures,' say the authors of the study. It should be noted that preservation is not unique to big bones. 'The preservation of primary soft tissues and biomolecules is not limited to large-sized bones buried in fluvial sandstone environments, but also occurs in relatively small-sized skeletal elements deposited in marine sediments,' the authors write. Meanwhile the fossil record, say the researchers, is capable of exceptional preservation. Further, tissues that are prone to decay, like skin and melanosomes (organelles containing melanin, the most common light-absorbing pigment found in the animal kingdom) are preserved as phosphatised remains or organic residues with considerable morphological fidelity. 'Yet, whether multimillion-year-old fossils harbour original organic components remains controversial, and, if they do, a positive identification of these biomolecules is required,' they say. Commenting on the analyses, the researchers say: 'Although infrared spectroscopy by itself cannot generally be used to identify specific proteins, it can nonetheless provide invaluable information on the molecular content in samples of unknown composition. Likewise, no other method employed herein stands alone (i.e. provides sufficient evidence for the survival of proteinaceous macromolecules over deep time).' Researchers from Midwestern University and Southern Methodist University in the United States contributed to this studyFor more information, please visit: Lund University:http://www.lunduniversity.lu.se/MAX IV Laboratory: http://www.maxlab.lu.se/PLoS(opens in new window) ONE:http://www.plosone.org/home.action
Countries
Sweden, United States