A regenerative therapy for hearing loss
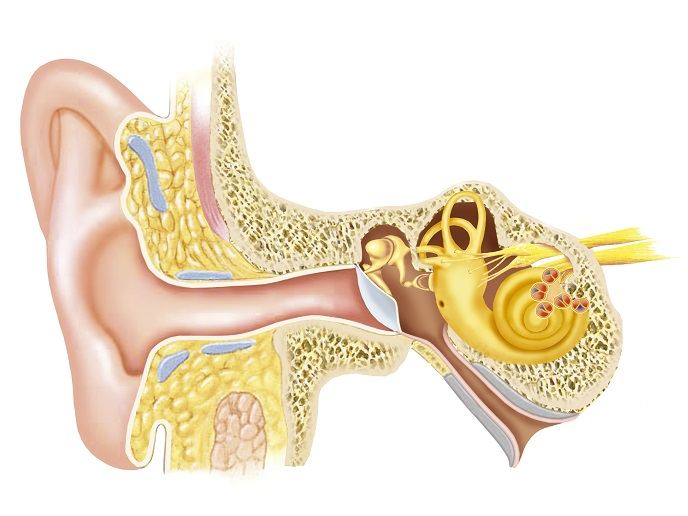
Hearing impairment is caused by the loss of cochlear hair cells(opens in new window), the sensory receptors of the auditory system. Hair cells receive sound waves in the inner ear and send signals to the acoustic nerve that connects the ear with the brain. Once damaged, hair cells will not regenerate spontaneously, and affected individuals are limited to using hearing aids or devices surgically implanted in the middle or inner ear. Hearing loss has an annual healthcare cost of over EUR 210 billion in Europe alone. To compound the situation, the hearing devices often perform poorly in noisy environments.
Testing gamma-secretase inhibitors (GSIs) for hearing loss
Animal studies have demonstrated that pharmacological inhibition of Notch signalling using gamma-secretase inhibitors (GSIs) can regenerate hair cells and restore their function. The EU-funded REGAIN(opens in new window) project was designed to take the GSI proof-of-concept to the clinic for the treatment of sensorineural hearing loss, the most common form of hearing loss. REGAIN pooled the expertise of seven partners in hearing loss biology, drug development and clinical trial design. “Our goal was to develop a suitable clinical formulation of the GSI and test it for the treatment of patients with hearing loss,” explains project coordinator and CEO of Audion Therapeutics(opens in new window), Rolf Jan Rutten. Partners worked extensively on product formulation development and tested it in preclinical studies (pharmacokinetics, toxicology). Following GMP manufacturing of the drug formulation, they received regulatory approval for conducting a clinical trial in patients with adult onset hearing loss. Delivery of the formulation containing the GS inhibitor to the middle ear takes place via a trans-tympanic injection, a procedure that is routinely used for delivery of drugs ear. From there, the GS inhibitor diffuses to the inner ear where the target cells are located, helping them to transform into new hair cells. The clinical study was conducted at University College London, University of Tubingen and the University of Athens. After showing safety and tolerability of the GSI, 44 patients were treated in a study looking at efficacy of the drug. Efficacy results demonstrated improvements in performance in several hearing tests in up to 35 % of patients, warranting further product evaluation.
Future prospects for REGAIN treatment
The REGAIN regenerative treatment for hearing loss addresses a significant, unmet medical need for millions of patients. Hearing loss in children hinders language learning and cognitive development, while progressive hearing loss in adults accelerates cognitive impairment and impedes social integration. “Development of pharmacological approaches to treat hearing loss can lead to a fundamental and significant improvement in the lives of people with this condition,” emphasises Jan Rutten. Although further development of the GSI inhibitor is required, the REGAIN project has helped shape the developmental and regulatory processes towards novel hearing loss treatments. Partners plan to conduct additional clinical studies in collaboration with other research groups active in the hearing loss field, hoping to have a product approved in clinical practice within 7 years. Furthermore, the project has opened the way for others in the field seeking to introduce treatment to the hearing loss patient community.