The next generation of targeted nanomedicines against cancer and infectious diseases
Gold-standard anticancer treatments, such as chemotherapy, are administered systemically and lead to toxicity-associated adverse effects. Nanoparticles offer an alternative means of delivering anticancer drugs safely and selectively to cancer cells.
Biodegradable nanoparticles for drug delivery to tumours
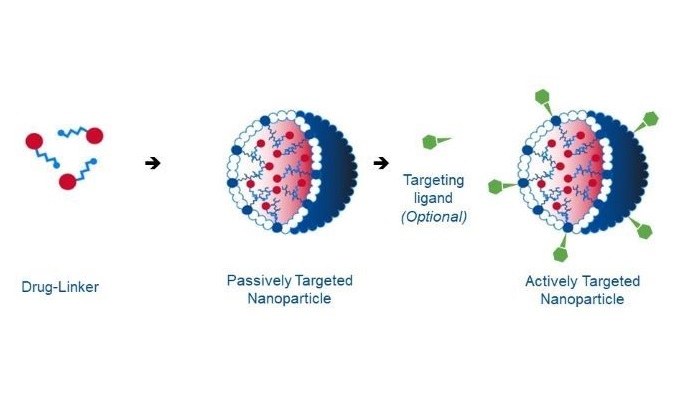
The EU-funded INTACT project has designed nanomedicines that increase efficacy and reduce toxicity of a given therapeutic by entrapping the compound in rationally designed nanoparticles. “Our CriPec® nanoparticles deliver the therapeutic agent to the target specific tissues, cells and intracellular components, with an improved pharmacokinetic and pharmacodynamic profile aiming for better therapies in oncology,” explains Axel Mescheder, INTACT coordinator and CEO/CMO of Cristal Therapeutics(opens in new window). CriPec® nanomedicines are based on proprietary polymeric nanoparticle technology and offer high loading, controlled stability, and controlled drug release. Importantly, the nanoparticles are fully biodegradable. A phase I clinical trial demonstrated that CriPec® nanoparticles exhibit prolonged circulation in the bloodstream, allowing them to passively target the tumour. Cancer tissue is known to accumulate macromolecules, such as liposomes, drugs and nanoparticles, at higher rate than normal tissues. This process is known as enhanced permeability and retention(opens in new window) (EPR) effect. EPR is caused by a leaky vasculature and a dysfunctional lymphatic system which does not allow nanoparticles to drain from the tumour site. During INTACT, partners undertook a phase II clinical trial of the specific drug candidate CPC634 in patients with ovarian cancer. CPC634 encapsulates the chemotherapeutic docetaxel(opens in new window) – widely used to treat a variety of solid tumours – in CriPec® nanoparticles. Results showed an improvement in neutropenia in the case of CPC634 compared to published data of systemically delivered docetaxel. Severe neutropenia(opens in new window) is an adverse effect observed in patients receiving docetaxel that can often lead to treatment cessation. Researchers also functionalised CPC634 with a positron emission tomography tracer PET tracer(opens in new window) based on Zirconium isotope 89Zr. This enables the monitoring of the biodistribution of CriPec® nanoparticles in real time. A clinical study using these radiolabelled nanoparticles confirmed the increased uptake of CriPec® nanomedicines into tumours.
Project significance and future directions
INTACT results validate the targeting concept for CPC634 in patients with cancer and the utility of the CriPec® nanomedicine platform in the field of theranostics and oncology. Apart from docetaxel, CriPec® nanoparticles can be employed to deliver other cancer therapeutics with reduced toxicity and increased targeting specificity. This opens the door to Cristal Therapeutics to collaborate with biopharmaceutical companies developing novel oncology drugs and continue to advance the CriPec® nanoparticle technology platform. Importantly, the non-invasive imaging approach developed during INTACT that uses radiolabelled nanoparticles can help analyse the biodistribution and tumour targeting of new candidates before they proceed down the drug development pipeline. “In addition to our approaches in oncology, Cristal Therapeutics has expanded its portfolio with the novel CriVac® vaccination platform, and is ready to engineer the vaccines of tomorrow,” emphasises Mescheder. This platform exploits the targeting specificity and safety profile, of the nanoparticles to activate the immune system. Overall, the CriPec® nanoparticles are expected to improve drug efficacy, increasing the quality of life of patients and offering a greater chance of prolonged treatment and survival. At the same time, they will reduce unnecessary treatments and associated costs.