Advancing the treatment of malignant glioma patients
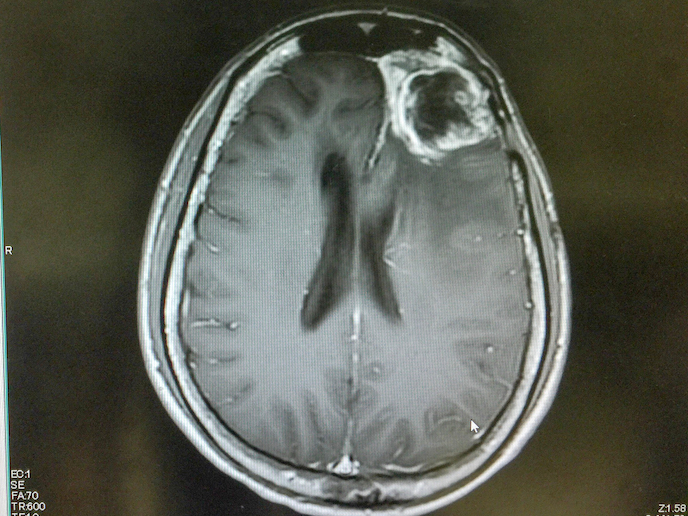
Glioblastoma is a rare and extremely aggressive type of brain cancer with a very high mortality rate. In fact, the average length of survival for most patients receiving treatment is a mere 15 months. But a promising new compound developed by Laminar Pharma(opens in new window) called LAM561(opens in new window) could help answer this unmet clinical need. LAM561 is a bioactive lipid that is inserted into the surface lipids of cancer cells, increasing the membrane fluidity and dislodging the key signalling proteins responsible for spreading the tumour. As a result, the lipid can enter the cell and regulate enzyme activity, altering the entire cell’s membrane composition and dissolving the cancer-associated lipid rafts. With the support of the EU-funded CLINGLIO(opens in new window) project, this novel therapy for treating patients with newly diagnosed glioblastoma is one step closer to possibly improving patients’ quality of life and life expectancy.
Unexpected challenges
The primary objective of the project was to demonstrate the safety and efficacy of the LAM561 compound within the established standard of care (radiotherapy plus temozolomide). To do this, researchers initiated a multicentre, international clinical trial. The purpose of this trial was to provide the assessment needed to apply for conditional marketing approval with the European Medicines Agency(opens in new window) (EMA). Indeed, LAM561 could be established as part of the standard of care for the disease if its addition to current treatments significantly reduces the time to tumour progression. But, as is often the case with complex clinical studies, conducting the trial proved more challenging than expected. For example, before researchers could even start, the EMA required a preliminary phase 1b trial to confirm the high safety profile and the most appropriate dose of LAM561 to be used in combination with radiotherapy and temozolomide. Although this additional trial seemed to delay the project’s timeline, it ended up leading to one of its most important outcomes: the confirmation of LAM561’s safety within the standard of care for glioblastoma.
Making lemonade
Ready to move to the phase II/III clinical trials, the project was again hit with an unexpected challenge. This time it was the COVID-19 pandemic. But here too CLINGLIO researchers took what looked like a lemon and made some lemonade. Specifically, the consortium(opens in new window) focused on areas that they could control, such as preparing for possible commercialisation and raising LAM561’s profile by publishing articles in high-impact scientific journals. The team also developed a new format for packaging the drug. Instead of glass bottles, they developed a sachet presentation that is more convenient, efficient and sustainable.
Clinical trial now under way
With COVID restrictions mostly lifted, Laminar Pharma is now continuing the necessary phase II/III clinical studies, with interim results on LAM561’s efficacy expected at the end of 2023. “Our hope is that these trials show LAM561 to be a better tolerated and more effective therapeutic approach than what is currently available,” says Victoria Lladó from Laminar Pharma. “This would be not only great news for a small biotechnology company such as Laminar but, more importantly, even better news for patients.”