Gene therapy for an inherited skin disease
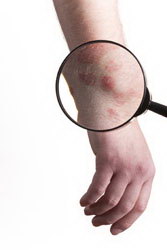
DEB is a rare, genetic, disabling skin disease caused by inherited defects of collagen type VII, affecting over 30,000 individuals in Europe. The skin of DEB patients is extremely fragile and prone to blistering and scarring, while it’s almost impossible to heal when wounded. Additionally, since collagen VII is associated with the epithelium of the esophageal lining, patients who are mainly children are very often malnourished due to trauma in the esophagus. Patient physical suffering can be compared to that of severely burnt people, while in the most severe cases affected individuals develop aggressive skin cancer and die prematurely. Although the genetic bases of the different clinical forms of DEB have now been elucidated, there is still no treatment for this debilitating group of diseases. With this in mind, the EU-funded ‘Gene therapy for Epidermolysis Bullosa: a model system for treatment of inherited skin diseases’ (Skintherapy) consortium aimed to develop a gene therapy protocol for DEB treatment. The approach was based on autologous transplantation of skin made in vitro using epidermal stem cells genetically modified to express COL7A1, the mutated gene in DEB. As a first step, project partners developed vectors and optimised gene transfer technologies for delivering the COL7A1 gene in epidermal stem cells ex vivo. These cells were then used to construct skin equivalents and assess the full morphological and functional reversion of the DEB phenotype. Pre-clinical assessment of the approach in vivo in animal models of cutaneous gene therapy was performed with the ultimate aim to be tested in phase I/II clinical trials. Translation of the Skintherapy approach into a clinical setting would require specially established protocols for generation of implant skin as well as production of clinical-grade viral vector preparations under GMP/GLP standards. Nonetheless, the generated model system exhibited overall efficient and safe delivery, making it an attractive technology applicable to many genetic skin disorders.