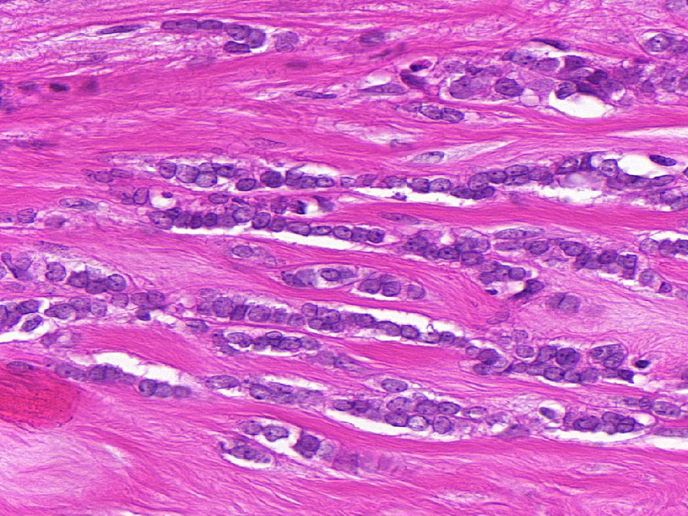
Precision diagnosis and treatment for difficult-to-treat cancers
Cancer genotyping has correlated the responses to targeted anti-cancer drugs with mutations in specific genes. Many of these mutations occur in signalling components, known as kinases. The difficult-to-treat subtypes of invasive lobular carcinoma (ILC) or triple negative (TN) breast cancer, which account for 25 % of all breast cancers, currently have a lack of targeted therapies, leading to poor patient prognosis. The EU-supported RATHER (Rational Therapy for Breast Cancer: Individualized Treatment for Difficult-to-Treat Breast Cancer Subtypes) project developed specific biomarker signatures to accurately predict outcome in patients with ILC (using the MammaPrint test) or response to therapy in patients with TN breast cancer (using the BRCAness test), as well as uncovered novel treatments for both breast cancer subtypes. RATHER’s results have immediate clinical potential to expand the reach of MammaPrint (an already approved diagnostic assay) into the subgroup of ILC breast cancers. The project has also contributed to several clinical trials, with the work yielding new diagnostic and therapeutic approaches, now paving the way for advances in breast cancer management. Tailoring of medicine Many cancers make changes to key cellular proteins, known as kinases. As well as playing a key part in physiological pathways, when it comes to cancer, kinases also act like molecular switches to control how it grows and spreads. RATHER investigated the genes that produce kinase proteins to determine if specific changes to the gene code, or other alterations in a patient’s cancer cells, can explain why the cancer forms and spreads. RATHER explored the rate of activation of all kinases (the ‘kinome’) in TN breast tumours lacking the oestrogen-, progesterone- and HER2 receptors (15 % of breast cancers) and ILC of the breast (10 % of breast tumours); in particular, 518 kinase genes were sequenced to identify mutations associated with either breast cancer. In addition to DNA sequencing, kinase alterations were also assessed at the mRNA and protein level, as well as for evidence of copy number variation. Alterations were examined using computer-based systems and pre-clinical lab models. As project coordinator Prof. William Gallagher explains: “Our work has identified differences in breast cancers of various subtypes and is based on the fact some of these alterations will turn out to be involved in driving the disease, not just random effects.” Where available, small molecule inhibitors were selected based on their ability to limit the growth of cells expressing subtype-specific kinase alterations. The RATHER consortium took a leading role in a multi-centre, Phase Ib/II clinical trial of a novel kinase inhibitor drug called ‘taselisib’. The initial Phase Ib trial successfully showed that a combination of traditional endocrine therapy (the drug tamoxifen) and this new kinase inhibitor was well tolerated by patients. As Prof. Gallagher summarises, “By uncovering changes to particular kinases and the function of the mutated protein, and then tracing that back to the patient’s specific breast cancer subtype, we discovered several new ways to accurately predict the outcome for patients and potentially also treat patients with this subtype more effectively.” RATHER contributes directly towards applications that will provide not only improved diagnostics, but also therapeutic options for breast cancer, one of the leading causes of cancer-related deaths in women. RATHER’s work aligns with wider efforts towards more individualised cancer treatment. The Phase II clinical trial of the novel kinase inhibitor is now underway alongside complementary trials.