Other therapies for mitochondrial diseases
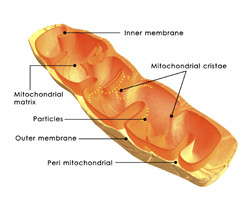
The cell contains little specialised powerhouses, the mitochondria, for producing the required energy for many cellular processes, including signal transduction. Mitochondria are distributed across the cell according to its function and energy requirements. Mitochondrial transport is itself an energy-demanding process and is carried out by the two cellular motor proteins dynein and kinesin. These two proteins attach to the mitochondrion and drag it along the cellular cytoskeleton. The key objective of the EU-funded DROMIT project was to investigate what affects the cellular transport of mitochondria in neurons. For this purpose, they studied primary neurons derived from the pupal stage of the fruit fly Drosophila melanogaster. The mitochondria in this model were manipulated to fluoresce green and thus visible under the microscope. DROMIT observations indicate that mitochondrial transportation speed and length are greater in the neuronal cell body than in neurites. This suggests that the process is space-dependent and that in confined areas such as the neurites transportation encounters resistance. The biochemical nature of this confinement in mitochondrial transport provides a basis for delineating its molecular background. A link with neurodegenerative disorders and mitochondria-associated conditions such as Charcot-Marie-Tooth disease should be made in future studies. Another part of the DROMIT project studied how mutations in the mitochondrial ATP-adenosinediphosphate (ATP-ADP) transporter stress sensitive b (Sesb) gene affect global gene expression. Sesb is homologous to the human ANT1 gene, which is mutated in the autosomal dominant condition progressive external ophthalmoplegia. Mutant flies could only perform the metabolic process of glycolysis, presenting with infertility and stress sensitivity. Amelioration of these phenotypes could be obtained either through genetic manipulation of the mitochondrial DNA or by boosting mitochondrial biogenesis. These solutions were also proposed for the treatment of mitochondrial myopathies and other human diseases associated with ANT1 deficiency.