Novel therapy for rheumatoid arthritis
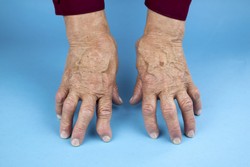
Rheumatoid arthritis is an age-related disorder responsible for significant pain and discomfort; in advanced stages it prevents patients from working. Current treatment relies on tumour necrosis factor alpha (TNF-α) blockers, a great cost to the healthcare system. TA101 is a small domain antibody currently under development for the treatment of rheumatoid arthritis and has successfully passed preclinical efficacy studies. It was engineered to specifically target TNF-α in patients afflicted with rheumatoid arthritis for monthly administration in combination with other therapeutics. The EU-funded TA101-GOCLIN (Clinical development of TA-101 for the treatment of rheumatoid arthritis) project wished to develop TA101 for clinical use and obtain safety data through Phase I clinical trials. The consortium comprised three small-medium enterprises from Portugal, Belgium and The Netherlands. To reduce the overall cost of treatment, partners aimed for a high-yield manufacturing process. They worked on an upscale method, optimising expression parameters, fermentation and purification conditions. However, efforts to upscale TA101 production were unsuccessful as the utilised expression systems produced insoluble antibody with significant aggregation issues. One of the partners developed an additional biological therapeutic within the TNF- α product category, which passed preclinical testing and reached the clinical development stage. This new compound provided a competent alternative to existing products on the market at a significantly reduced cost. To ease drug administration, project partners developed an innovative device that relied on ceramic nanoporous microneedle arrays. These microneedle patches enabled autonomous drug administration through the skin without the pain of conventional injections. Importantly, the device proved suitable for many protein-based drugs apart from antibodies, and self-administration at home reduced the need for patient hospitalisation and treatment costs. Taken together, the new drug delivery approach is expected to enter the growing market of biological therapeutics and facilitate the administration of drugs for different diseases.