Mechanistic insight into endocytosis
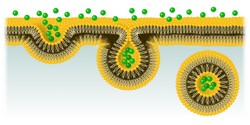
The cell membrane represents the interface between cells and their microenvironment, driving a number of vital processes such as cell to cell communication, attachment to the extracellular matrix and movement. For uptake of nutrients and signalling molecules, cells employ a specialised internalisation and trafficking pathway known as clathrin-mediated endocytosis (CME). Although deregulation of CME is implicated in various diseases, the mechanism regulating this process remains poorly understood. Scientists of the EU-funded CME-REG project worked to delineate the mechanism that triggers this process by focusing on key core components: the plasma membrane lipids, the cargo to be trafficked and adaptor proteins. “Our aim was to dissect the steps of endocytosis and identify the key regulatory determinants that could also be implicated in some disease associated with cell signalling failure,″ explains project coordinator Dr Zuzana Kadlecova. Regulation of CME During CME, the nutrients or macromolecules to enter the cell bind to receptors on specific regions of the outside part of the cell membrane. Subsequent engulfment takes place in small vesicles coated on the outside with the protein clathrin. Live cell imaging shows that the process of CME commences with the localisation of clathrin and other adaptor proteins on the cytoplasmic face of the membrane in clathrin-coated pits (CCP). To capture and analyse the dynamics of the CME process, scientists developed a workflow employing quantitative live cell fluorescence imaging of the basal membrane in cells that have small alterations in the sequence of the main endocytic protein, AP2. AP2 is one of the most evolutionary conserved proteins from yeast to human and earlier biochemical studies reveal that AP2 complexes have a well-established role in initiating CCP assembly. Intriguingly, AP2 can instantly transform from one shape to a different one and simultaneously change its function and location. However the hierarchy of the steps implicated in the switching of AP2 to its active mode, ready for action and cargo capture, remained enigmatic until now. Through live-cell microscopy analyses, researchers were able to visualise thousands of CCPs at a time. Sophisticated particle tracking algorithms and mathematical analyses were also employed to dissect the stages of CME and the sequential conformational changes that activate AP2 to initiate and stabilise CCP formation. The team discovered that cargo binding to AP2 is absolutely necessary for stabilising the growing pit at the plasma membrane as well as its maturation. Cells with mutations in specific AP2 subunits exhibited significantly lower polymerisation of clathrin at CCP sites, clearly underscoring the importance of specific binding sites for AP2 activation. CME-REG findings clearly support the model of AP2 conformational change, where the protein alternates between a close, cargo-inaccessible state to an open active conformation. Project results show that AP2 regulation drives these conformational changes and is responsible for stabilising nascent CCPs. Impact of the results Using a highly interdisciplinary approach, CME-REG scientists managed to decipher the molecular mechanisms underlying the regulation of the early stages of CME. Dr Kadlecova unravelled in real time how AP2 orchestrates cargo selection for internalisation and its role as a conductor or CCP assembly from its inception to the end. Although project findings were generated at the cell level, they can most likely be extrapolated to the in vivo situation. Given the indispensable role of AP2 in normal embryonic development, results also offer fundamental knowledge on events early on in life. Importantly, they provide the basis for understanding the molecular aetiology of many diseases associated with impaired endocytosis.