Innovative device delivers eye injections for sight degeneration
In AMD, retinal cells in the macula – the point of sharpest vision – gradually die, making everyday activities such as reading and driving difficult. Regardless of the pace with which the condition develops, late stages lead to irreversible vision loss unless treated. Since 2006, treatment against vascular endothelial growth factor (VEGF) has dramatically changed AMD outcome by stopping abnormal blood vessel growth in the eyes of patients. Drug administration involves intravitreal injections, a method that is in need of optimisation due to patient discomfort.
A device that stabilises intravitreal drug injections
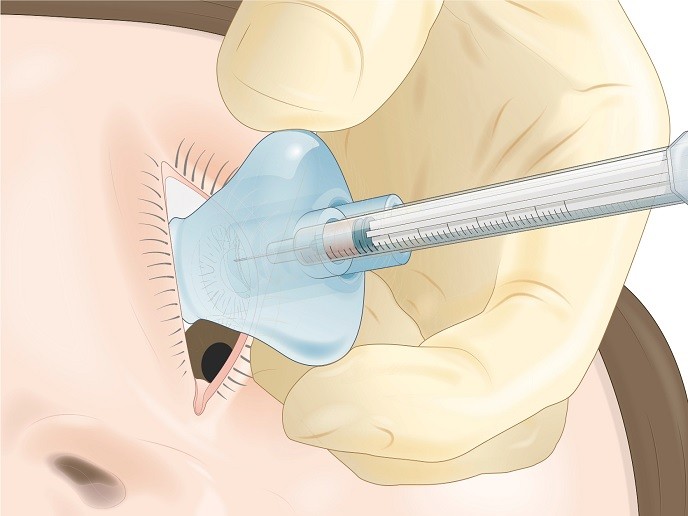
To address this issue, the EU-funded Vitreoject project developed an innovative device for making the procedure more efficient and comfortable for the patient. “Our goal was to increase access to eye care by improving and optimising process operations,″ explains project coordinator Robert Kromer. The ‘introducer’ as they call it, integrates all perioperative steps into a single tool, simplifying and streamlining the injection process. The doctor only has to attach the desired medication to the device and inject without further preparation. Existing solutions in the market mostly focus on stabilisation and positioning assistance during the injection. While these features are relevant, the needed devices are add-on products to the current process. The Vitreoject introducer delivers stability in the procedure and stands out as it facilitates localised sterilisation. The medication needle and disinfectant solution are easily inserted into the Vitreoject device and the flexible grip aid is compressed to create a vacuum, which gives the patient a reassuring feeling of stability, important in such a sensitive area. Following localised sterilisation of the conjunctiva, advancement of the needle is guided without additional aids such as an eyelid barrier used traditionally. This achieves efficient treatment through an optimally adapted workflow with maximum safety and patient comfort. The original technology concept was formulated in late 2017, and a patent was filed in 2018. Vitreoject developed the device with constant feedback from clinical experts. The functional working prototype has been validated in an experimental setting using post-mortem porcine (pig) eyes with very good results.
Device commercialisation prospects
During the Vitreoject project, partners reviewed the feasibility of the device mainly in terms of market size, market opportunity, market approval and technical specifications. They also identified current and future competitors that manufacture similar products. Using a bottom-up analysis, they developed a pricing strategy for the final device. Kromer emphasises that “the substantial support from the EU allowed us to grow our network, especially in terms of experts in medical product certification, intellectual property, manufacturing, quality control, and commercialisation strategy.″ Collaboration with industry has enabled the identification of product strengths and limitations, moving from 3D-printing to device manufacturing in the near future. Currently, more than 90 million injections into the eye are performed worldwide not only for AMD, but for other vision-threatening diseases like retinal vein occlusion or diabetic macular oedema. Considering that AMD risk reaches 30 % in people over 75, there is a substantial niche for intravitreal injection devices. In view of the future, Kromer is confident that “implementation of the Vitreoject device has the potential to save nearly EUR 250 million annually in healthcare costs in Europe by reducing operating theatre time.″