State-of-the-art quantitative MRI for early diagnosis of chronic liver disease
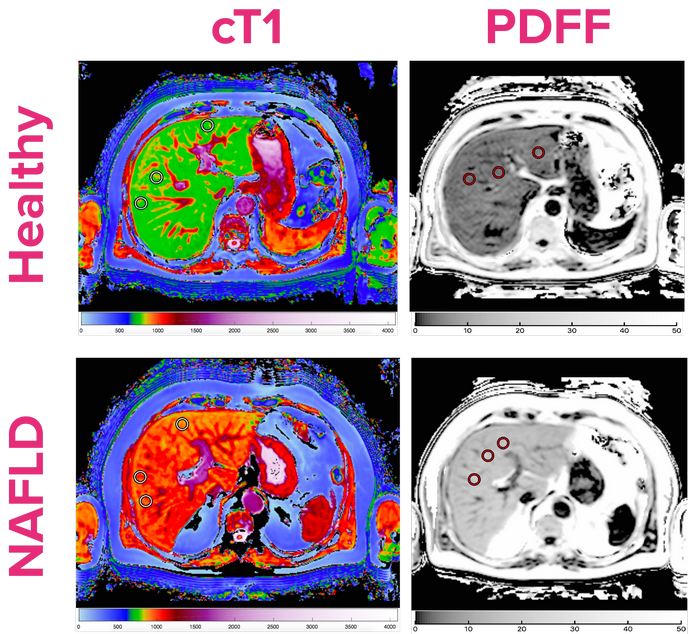
Non-alcoholic fatty liver disease (NAFLD) is incredibly common due to changes in diet and activity levels. NAFLD often goes undiagnosed and subsequently untreated for some time. Advanced forms of the disease can lead to non-alcoholic steatohepatitis (NASH) with further progression to cirrhosis and liver failure, a huge socio-economic burden. If detected in its earlier stages however, disease can be reversible. Currently, chronic liver disease is diagnosed with a liver biopsy, which is costly and invasive, and cannot meet the demand of this prevalent disease. Early identification of liver disease in a non-invasive and cost-effective manner is key to improving quality of care and patient outcomes.
A new test, a new dawn
LiverMultiScan®, a novel tool that combines quantitative MRI with advanced image analysis, addresses these issues. It has been shown to predict clinical outcomes in patients with a variety of liver conditions and correlate with liver biopsy results. LiverMultiScan® has a lower cost than liver biopsy and has been endorsed by Blue Cross Blue Shield, the largest payer group in the United States, following FDA clearance. In the EU, LiverMultiScan® is CE-marked, and has been adopted by large academic consortia (e.g. LITMUS)(opens in new window). The EU and industry-funded RADIcAL(opens in new window) project is conducting two trials to compare LiverMultiScan® performance against the standard care pathway for assessing chronic liver disease, in a bid to embed LiverMultiScan® in EU clinical guidelines. Based on technology patented by founders of the coordinating company, Perspectum, LiverMultiScan® represents an exciting step in the care for liver patients who may no longer need to get biopsied for diagnosis or disease monitoring. LiverMultiScan® can be deployed on any modern MRI scanner with data processed centrally. Standardisation of LiverMultiScan® metrics ensures that measurements are equivalent across different MRI scanners and magnetic field strengths.
Lessons from RADIcAL: Optimising diagnostic pathway for chronic liver disease with LiverMultiScan®
The RADIcAL project has shown that in a European cohort of 135 patients with suspected NAFLD, nearly a quarter of the patients were identified as low risk for developing more serious forms of liver disease with potential to avoid unnecessary future clinical appointments. RADIcAL partners are also investigating the effectiveness of LiverMultiScan® against gold standard liver tests to assess liver health in adult and paediatric liver transplant recipients. RADIcAL’s innovation has highlighted how LiverMultiScan® can be used for early identification of patients at risk of poorer health outcomes. This is expected to reduce liver transplant surgery and overall healthcare costs and benefit patients. “A cost-effective tool for non-invasive liver tissue characterisation would be a major step forward in the treatment of patients with liver diseases such as NAFLD,” emphasises the principle RADIcAL investigator from Leiden University Medical Centre, Minneke Coenraad. According to Rajarshi Banerjee, CEO of Perspectum: “There is a real opportunity here, not only to make significant economic savings at a time when all health services are under pressure, but also to reduce the number of patients having to undergo what can be a stressful and painful procedure.”