Medicine’s bubble trouble popped by innovative valve
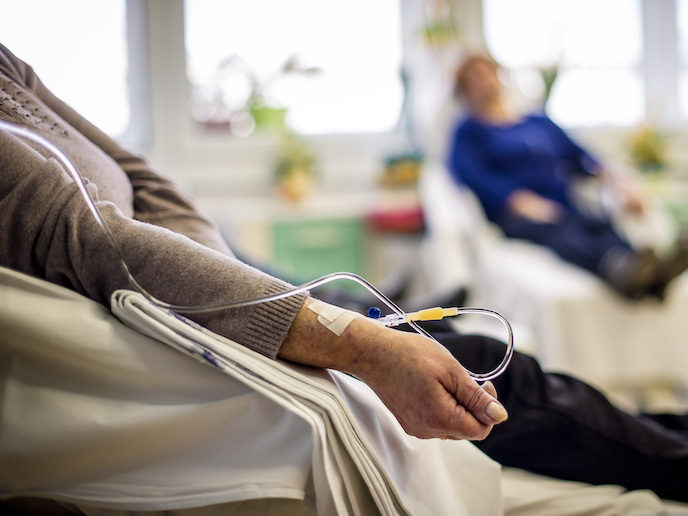
Intravenous (IV) infusion of fluids and medicine is the most common invasive medical procedure, with 90 % of hospital inpatients receiving one(opens in new window) and more than 2 billion performed globally every year. Yet each of these carries a serious health risk if bubbles of gas are allowed to pass from the infused fluid to the patient. Air embolism occurs when bubbles pass into a patient’s bloodstream and disrupt the flow of blood, leading to adverse events including complications of the vascular system, heart or brain. It is classed as a ‘never event’(opens in new window): a largely preventable medical error which should never be allowed to happen.
Non-intervention
Although clinicians will carefully clear the line when setting up the IV drip, air can enter when the line is broken, for example to inject a drug. The EU-funded Gasgon project investigated the market feasibility of a novel device that can prevent air bubbles entering a patient’s bloodstream and so avoid these complications. “Depending on the nature of the fluid, some can degas, as a result of a reaction between the drug and the fluid,” adds Gasgon project coordinator Vincent Forde. “Cold fluids from fridge degas more easily as they warm up, just as bubbles in water are released when a kettle warms up.” Existing solutions include: “Observational alarm systems on IV pumps that recognise bubbles, stop the flow and sound an alarm,” says Forde, director of Gasgon Medical(opens in new window), which hosted the project. The problem with these, he explains, is that they require the intervention of a doctor or nurse to clear the line, making them a resource-intensive solution.
Toxic drugs
Forde’s low-cost solution doesn’t require observation or intervention. “We created a disposable device that attaches simply onto any IV line. It’s fully mechanical, non-powered, functions in all positions and orientations, and works with movement of the patient,” he notes. Support from the EU allowed Forde and his colleagues to examine where Gasgon could most effectively enter the market. “This gave us the opportunity to engage with more nurses and doctors, identifying high-risk areas. We saw an opportunity in the infusion of hazardous drugs, such as with chemotherapy used in oncology.” Clinicians are exposed to minute amounts of toxic chemicals when they install and manage IV lines containing chemotherapy treatments. This occupational hazard results in serious fertility issues, cancer and even death. The EU has a new carcinogens and mutagens directive which is designed to protect workers from exposure to these hazardous drugs.
Clinical trials
“Our closed-system aspirator device can capture chemical vapours in a sealed chamber, so the nurse doesn’t have to open the line, and the patient receives the full intended dose without need for interruption,” explains Forde. “Our technology can reduce exposure to the nurse, while improving dose accuracy for patients and delivering significant cost benefits to healthcare providers.” Next, the company plans to raise more funding while also running a trial of the safety vent in hospitals in Portugal and Spain, as part of a move toward obtaining a CE mark for the device. With over 1 million infusions of hazardous chemotherapy every day, the opportunity for impact is large. “Assuming our plans follow through, we’ll see market entry in 2022,” says Forde.