Unveiling the regulatory architecture of lysosomal gene transcription
Emerging evidence indicates that the transcription factor EB TFEB(opens in new window) plays a central role in the transcriptional regulation of lysosomal genes. TFEB binds onto specific sequence motifs encountered in the promoter of lysosomal genes and ensures their coordinated expression. Therefore, deciphering the mechanism of TFEB activity regulation is central for comprehending how cells adapt to a wide variety of internal stresses and environmental fluctuations.
TFEB activation from a different angle
Investigation of TFEB activity has mainly focused on its post transcriptional modifications and in particular its phosphorylation state which seems to determine its subcellular localisation and function. Under nutrient-rich conditions, TFEB remains in the cytosol with no transcriptional activity. Under nutrient restriction, TFEB is no longer phosphorylated and can translocate into the nucleus where it acts as a transcription factor. The scope of the EpiTFeb project was to explore TFEB activity from a different angle, by defining the regulatory architecture of TFEB activation in different cellular environments. The research, undertaken with the support of the Marie Skłodowska-Curie (MSC) programme, initially employed an in-silico analysis to identify TFEB regulatory elements and predict transcriptional regulators. “The project was designed based on the main hypothesis that TFEB transcription dynamics represent a key determinant for its activity and could synergise with its established post-translational regulation,” explains the MSC research fellow Marcella Cesana. The research team undertook a gain-of-function approach, combined with a luciferase-based(opens in new window) assay, to test various candidate transcription factors. They also performed a functional validation of the identified TFEB transcriptional regulators by evaluating the effect of their modulation on gene expression and, more generally, their involvement in TFEB-mediated cellular processes.
The role of TFEB in autophagy and cancer
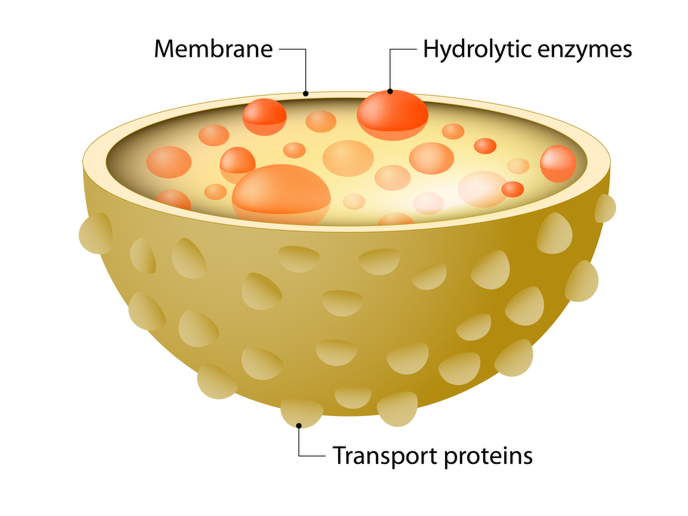
EpiTFeb results showed that TFEB transcriptional dynamics are highly variable across different cellular systems and nutrient conditions. Scientists determined the regulatory elements that drive TFEB transcription and delineated the molecular mechanism underlying TFEB transcription upon nutrient starvation. Collectively, EpiTFeb findings unveiled the transcriptional landscape of TFEB and most importantly provided a novel and yet unexplored angle to revisit its contribution to diseases associated with impaired lysosomal function. Lysosomes are implicated in autophagy(opens in new window), the ‘self-eating’ recycling process which degrades aggregated or misfolded proteins, aged or defective organelles and intracellular pathogens. Disturbances in autophagy have been documented in cancer, with TFEB displaying a pathogenic role. As a result, TFEB seems to affect cancer cell survival and energy metabolism by regulating lysosomal biogenesis, autophagy and other pathways known to contribute to carcinogenesis. According to Cesana, future plans include “the investigation of the mechanism by which the oncogenic process leverages the lysosome dependent degradative pathway for its survival by activating TFEB expression.” To achieve this, Cesana will select specific cancer models in which TFEB activity is dysregulated due to the altered of the TFEB transcriptional regulators or because of altered DNA accessibility at its regulatory regions.