Targeting nervous system enzyme pathways
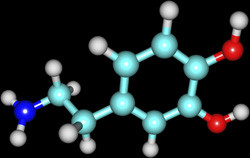
Enzymes are nature's catalysts, proteins that facilitate a myriad of chemical reactions in cells and affect many different functions. Cu-containing enzymes are critical to energy transformations and the synthesis of neurotransmitters – chemicals mediating nervous system signal transduction. Motivated by the potential to find targets for new drugs, EU-funded scientists launched the project ITCSCEN. They studied two of the most important Cu-containing enzymes that catalyse the hydroxylation of substrates involved in nervous system function. Peptidylglycine alpha-hydroxylating monooxygenase (PAM) and dopamine beta-monooxygenase (DBM) are binuclear Cu proteins containing two Cu atoms. PAM catalyses a modification to more than half of all peptide neurotransmitters that increases their function and longevity. DBM has many nervous system functions, among which is to catalyse the conversion of the neurotransmitter dopamine to the neurotransmitter norepinephrine. Catalysis in both pathways begins with Cu reduction and oxygen activation. This leads to a sequence of reactions including hydrogen abstraction, water binding and hydroxide transfer to the substrate. Despite extensive research, the mechanisms of action have been unclear. Scientists employed a combination of spectroscopic and computational methods to study each step in the reaction in depth. Analyses evaluated geometric and electronic structures of the reactant complexes, transition states, reaction intermediates and products within the enzyme environment. They also studied the effects of conformational changes on reaction mechanisms using computer simulations. ITCSCEN results have shed important light on the mechanisms of action of two binuclear Cu enzymes with important roles in nervous system function. Detailed understanding of the structure and function of components along the catalysis pathway should guide targeted drug development for devastating nervous system disorders.