Zebrafish in the fight against tuberculosis
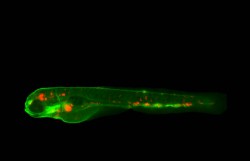
Currently, treatment of TB entails administration of a course of chemotherapy drugs. However, to overcome drug resistance issues, novel therapeutic approaches are being considered including host-targeted treatments. A better understanding of the innate immune response and the mechanisms that the pathogen uses to manipulate host defence could increase the chances of improving existing therapeutic strategies for TB. Autophagy has recently emerged as a crucial host defence mechanism known to counteract the ability of Mycobacterium tuberculosis to survive inside host cells. Through an autophagy-mediated mechanism, bacteria are targeted for degradation. Autophagy is also thought to control inflammatory responses but the interaction between inflammation and autophagy in the host defence against TB remains unclear. Towards this goal, the EU-funded Inflammafish(opens in new window) project employed the well-established zebrafish model of TB to study inflammation and autophagy, two fundamental processes critical to TB pathogenesis. Autophagy and inflammation during infection “The aim of the Inflammafish proposal was to gain insight into the mechanisms of host defence against TB and identify new targets for therapy,″ explains project coordinator Prof. Annemarie Meijer. To this end, the scientific team used zebrafish as it constitutes an excellent tool for visualising these processes in vivo. Previous work by the group had discovered that the DNA damage-regulated autophagy modulator (DRAM1) protects against TB in the zebrafish model. Scientists also implicated this important autophagy regulator in inflammation, as they found that it strongly affects expression of interleukin-1beta. Intriguingly, it emerged that deregulation of one of the pathways is enough to affect the outcome of Mycobacterium tuberculosis infection. When the autophagy host response is deficient, the bacteria progress inside the host. Importantly, scientists observed that the pathogen can manipulate the inflammatory response for its own benefit, increasing the spreading of the infection by inducing pro-inflammatory death of infected cells. Manipulation of one of the key proteins of the inflammatory pathway can thus help tackle the infection. Towards novel therapies “Collectively, our results showed that the inflammatory response and autophagy are both activated and necessary for an effective defence during TB infection,″ outlines Dr Monica Varela, the Marie Curie research fellow carrying out this project. Ongoing work focuses on the interplay of these pathways with different cell death mechanisms to determine the fate of infected macrophages and thus the outcome of infection. Furthermore, the Inflammafish team is keen to study the genome-wide effects of autophagy modulation on the inflammatory response, which will help identify novel targets for TB therapy. Future research efforts will focus on developing new strategies for treatment of TB using chemical modulators of the autophagy and inflammation pathways. Advancing current knowledge on host regulatory pathways that could potentially be manipulated will help develop new treatment possibilities for TB as well as other pathologies with similar characteristics. Prof. Meijer envisages “future anti-TB drugs to intervene on the host-pathogen interaction, overcoming resistance issues associated with current treatment modalities.″