A safer medication for treating eczema
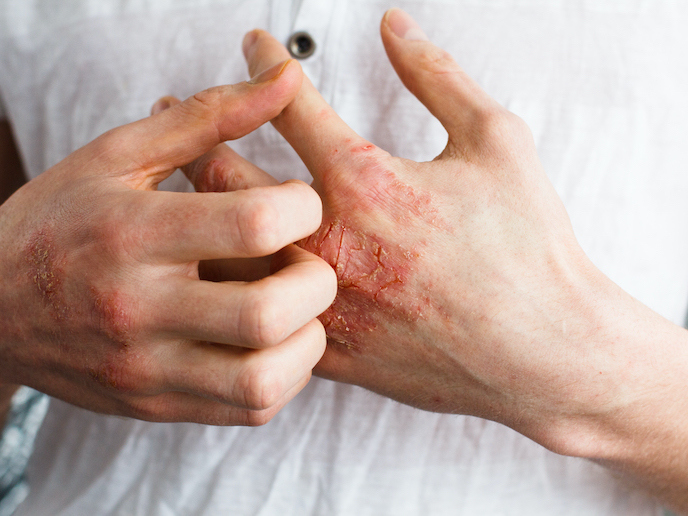
Atopic dermatitis, also known as eczema(opens in new window), is a chronic skin condition that typically develops in early childhood. According to the American Journal of Managed Care(opens in new window), eczema cases have increased two- to three-fold in industrialised countries, impacting between 15 % and 20 % of children and 1 % to 3 % of adults worldwide. The disease’s main symptom is an itchy rash, although severe cases can result in infections and skin damage. Eczema is typically treated by avoiding certain irritants, such as soap, and using moisturisers and steroid-based creams. However, the long-term use of steroids can cause permanent stretch marks, bruising, discolouration and the formation of spider veins, as well as a thinning of the skin. That is why TIRmed Pharma(opens in new window), a Swedish pharmaceutical company, has developed a non-steroid cream. “Our non-steroid medication targets mild to moderate eczema-related itching and is able to dampen inflammation by selectively interfering with the body’s immune system,” says TIRmed Pharma CEO Anna-Lena Spetz. “Furthermore, our solution has an excellent safety profile, is affordable, and can be easily self-applied.” Now, thanks to the support of the EU-funded TIR-C project, TIRmed Pharma is one step closer to bringing its innovative treatment to market.
Assessing the product’s clinical feasibility
The main objective of the TIR-C project was to assess the clinical feasibility of TIRmed Pharma’s non-steroid cream. This involved ongoing discussions with contract research organisations and clinical investigators to confirm the clinical development plan. It also meant confirming the regulatory route and required clinical endpoints needed to gain market approval. “This part of the study included a cost and risk assessment for every step of the technical and clinical development process,” explains Spetz. “We also developed strategies to extend our collaboration with clinical, distribution and pharma partners.”
From one to two
One unexpected outcome of the project was the discovery of a new product pipeline. “Research results showed that our compound has an antiviral component, so TIRmed’s business plan now entails two separate products,” adds Spetz. “In addition to our topical treatment for mild to moderate eczema, called TIR-C, we’re also developing TIR-A, a nasal formula for treating respiratory tract infections.” The latter was recently licensed for use in the EU-funded Fight-nCoV project. That project is testing the effect of intranasal-delivered antivirals against SARS-CoV-2, the virus behind the COVID-19 pandemic.
Two well-positioned products
With the support of EU funding, TIRmed Pharma now has not one, but two innovative medical solutions. Backed by strong business plans, both solutions are well-positioned to attract investors. “We are currently negotiating with investors to finance TIRmed’s activities and securing the necessary patents,” concludes Spetz. “This includes moving both TIR-C and TIR-A into pivotal proof-of-concept studies and securing the necessary financing to conduct preclinical testing.”