Smart artificial muscles against venous disease
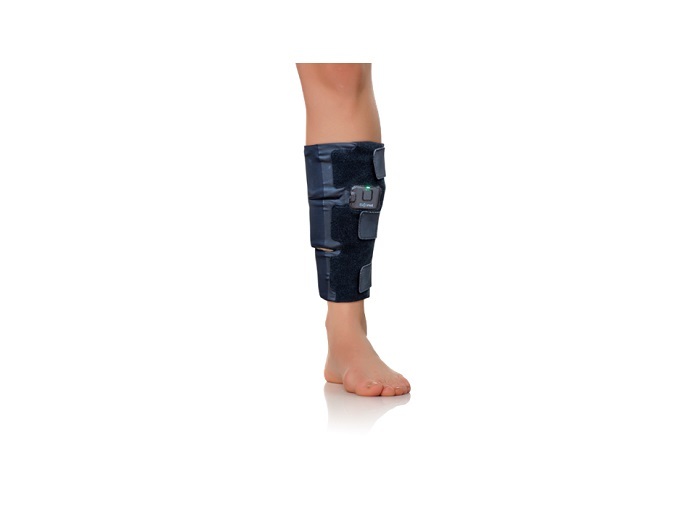
Poor blood circulation is responsible for venous diseases (VDs)(opens in new window), such as oedema, varicose veins, lymphedema and multiple sports injuries, which are painful and negatively impact patients’ quality of life. Compression stockings are the gold standard treatment for VDs, used to apply a constant pressure on the lower limbs. However, they are very difficult to apply, uncomfortable and have limited effectiveness, leading to low compliance. A much more expensive alternative are pneumatic compression devices. These devices use an inflatable sleeve (garment) to massage the patient’s legs, mimicking rhythmic calf muscle contractions. Today’s garments are cumbersome and have noisy compressors and heavy air pumps, which limit the patient’s mobility and cause discomfort.
Novel materials that mimic muscle contraction
To address these shortcomings, the EU-funded ComFyt project designed a unique product that offers effective compression therapy with complete mobility, high comfort and ease-of-use. “Our device is based on a proprietary technology that utilises innovative materials which continuously massage the legs throughout the day to stimulate circulation,” explains Omer Zelka, Founder and CEO of ELASTIMED(opens in new window) that developed the ComFyt device. Partners employed electroactive polymers(opens in new window) (EAPs) also known as Artificial Muscle, materials that can change in shape and size once stimulated by an electric field. They developed a scalable process that allowed the production of thin EAP components for numerous healthcare devices. ComFyt integrates these Artificial Muscle bands into an active compression device that wraps around the leg and mimics the rhythmic calf muscle contractions to promote fluid movement in the leg. The controlled active sequential contractions constitute a highly effective clinical treatment. This was demonstrated in a clinical study on 11 healthy subjects, where the ComFyt device showed an average increase of the peak flow velocity in the popliteal vein(opens in new window) by 141 % after 30 minutes of usage. The second-generation device features double the pressure range as well as reduced thickness and weight.
Advantages and future prospects
The ComFyt device demonstrated a comparable outcome with standard pneumatic compression. However, their cumbersome design and noisy compressors limit patient mobility and cause discomfort. In contrast, the ComFyt EAP actuator is small, flexible and low in energy consumption, rendering the technology ideal for future medical and non-medical application. Importantly, user experience questionnaires during the clinical trial demonstrated a much higher comfort level compared to pneumatic compression devices. Millions of people worldwide suffer from painful and swollen legs due to poor circulation. In extreme cases, chronic compromised circulation may even lead to deep vein thrombosis. The unique capabilities of ComFyt alongside its sleek, battery-powered, easy-to-wear and comfortable design are expected to improve the quality of lives of people suffering from venous and lymphatic diseases. Moreover, the innovative Artificial Muscles can be applied in a number of additional applications in the future, including compression devices for different body parts. “In this high-potential yet high-risk venture, the Horizon 2020 grant helped overcome the technological uncertainty of ComFyt,” emphasises Zelka. The company has begun the process of raising its next round of financing to perform a clinical study on patients, apply for regulatory approval and commercialise ComFyt in early 2022.