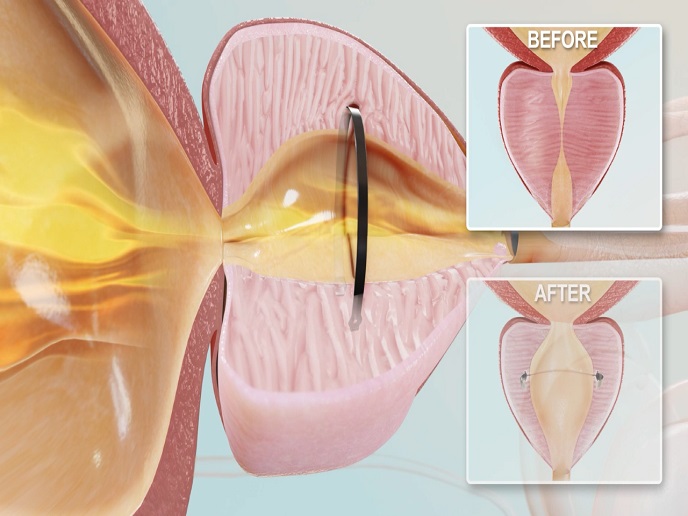
A reshaping ring to treat enlarged prostate
The prostate is a reproductive gland that surrounds the urethra in men. Along with seminal vesicles, it helps produce semen, the reproductive viscous fluid required for sperm to travel.
Benign prostatic hyperplasia: no definitive risk-free treatment
As men age, the prostate gland enlarges, leading to a common condition known as benign prostatic hyperplasia(opens in new window) (BPH). Nearly half of men aged 50 have BPH: urinary tract symptoms include changes in urinary frequency, incomplete voiding of the urine bladder, nocturia and sexual dysfunction. BPH can be managed for several years using chronic medication, but ultimately a prostate treatment will be recommended to prevent further complication. Current treatments like prostate tissue removal, urethral stents and mechanical prostate lifting either provide short-term relief or are associated with side effects.
A prostate ring to prevent urethral constriction
To address this problem, the EU-funded ClearRing project developed an implant in the shape of a ring. “It is a minimally invasive solution that embeds within the prostate tissue to expand the restricted urethral area, offering relief for many years,” explains Gilad Hizkiyahu, project coordinator and CEO of ProArc Medical(opens in new window). The ClearRing system consists of an implant and a delivery device. The former, super-elastic and biocompatible, winds around a dilatation balloon at the distal tip of the delivery device. The proximal side of the device features an ergonomic control handle, a connector for balloon inflation and a diathermy(opens in new window) unit. The ClearRing is inserted into the patient's urethra and visually guided until it reaches the prostate. Following inflation of the balloon to expand the urethral path, the device performs a tiny incision using diathermy(opens in new window). This facilitates release of the implant inside the prostate tissue supporting the urethral walls from collapse. The entire procedure takes less than 10 minutes under reduced anaesthesia.
Clinical efficacy and durability
The first generation of the ClearRing device has been tested in a clinical trial, resulting in alleviation of patients’ symptoms and reduction of prostate hyperplasia. An improvement in measured urine parameters and quality of life were also observed. Clinical trial results put ClearRing next to the gold standard treatment in terms of efficacy – and at a lower cost. “A BPH treatment should be safe and effective, but also provide the patient with long-term symptom relief; no patient wants to hear they have to repeat the procedure in a couple of years’ time,” emphasises Hizkiyahu.
Next-generation device slated for market
ProArc is getting ready for its multicentre pilot study, aiming to prove safety and efficacy of its latest generation of visually guided implant-embedding delivery device. The company will then apply for regulatory approval and launch a large-scale global pivotal study that will hopefully establish ClearRing as a standard-of-care treatment for BPH. ClearRing is expected to reach the market in 3 to 4 years, after extensive clinical trials. The nature of the procedure will improve patient compliance and overcome the need for chronic medication. It will help millions of men worldwide solve their BPH problem rather than avoid treating it due to fear of adverse effects.