New tailored approach signals a breakthrough in directed evolution of stable enzymes
Enzymes found in nature could be tweaked to perform new tricks by altering their DNA sequence. The glimmering of this idea, which earned an American chemical engineer the 2018 Nobel Prize in Chemistry(opens in new window), came from Darwin’s conception of biological evolution that combines the processes of genetic mutation and recombination, and natural selection. This evolutionary process is now increasingly being employed in chemical laboratories to create new enzymes: The process begins with finding an amenable enzyme that weakly performs the desired reaction that is then mutated and tested for the desired activity. After many optimisation cycles, an evolved enzyme with the desired activity wins out. This artificial, speeded-up version of the evolution process is called 'directed evolution'. Enzymes developed using directed evolution are a green alternative to toxic metals or large amounts of organic solvents. Despite progress in the field, the use of enzymes for producing fine chemicals, materials and drugs is not fully harnessed due to their limited tolerance to non-physiological conditions. The elevated temperatures or unnatural microenvironments (such as extreme pH and the presence of organic solvents and detergents) often prove unsuitable to the enzymes, compromising their stability and thus their selectivity and activity.
Circular proteins are more stable
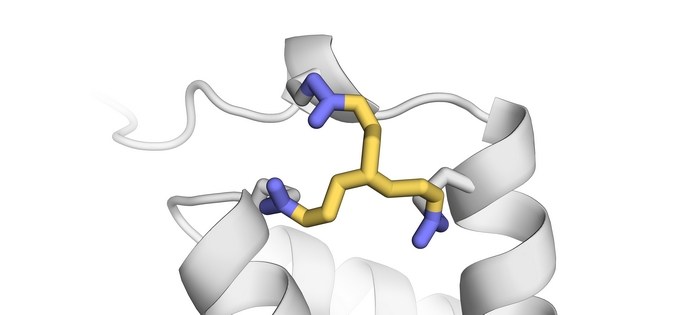
European scientists tackled the topic with support of the European Research Council (ERC). Professor of chemistry at the Department of Chemistry and Pharmaceutical Sciences(opens in new window) at the VU Amsterdam Tom Grossmann and his team have synthesised and tested cross-linking reagents with diverse physical and chemical properties that bind to different parts of the enzymes to produce rugged and more efficient versions. At the core of their work is an entirely new stabilisation strategy called 'in situ cyclisation of proteins' (INCYPRO). Grossmann explains the principle behind the concept: “INCYPRO enables a straightforward, computational and non-iterative design process that involves the introduction of three cysteines into the enzyme, which then react with an electrophile cross-linker with three centres.” The project team created different cross-linkers by ‘decorating’ a symmetric core structure – triethylamine, triazinane or benzene – with three electrophiles – acrylamide, chloroacetamide and vinyl sulfonamide (chemical species which attract electrons). The cross-linker reacts with the three thiol side chains of the cysteines on the enzyme, resulting in a multicyclic protein that has a more robust (stable) core structure. The more ‘electron-loving’ the electrophile, the faster the cross-linker bonded to the thiol groups.
A better alternative to existing stabilisation approaches
INCYPRO utilises enzymes entirely composed of natural amino acids that can be obtained rapidly, cost efficiently and in large quantities. “Current enzyme stabilisation approaches require multiple optimisation cycles or involve non-natural amino acids which complicate enzyme production. This prolongs the design process and increases production costs of the new enzyme,” adds Grossmann. What’s more, an existing approach focuses on increasing the stability of a small part of a protein. Grossman and his team continued the work conducted by another EU-funded project, PEP-PRO-RNA, also supported by the ERC. “In PEP-PRO-RNA, we developed molecular architectures (cross-linkers) to connect two or three different parts of a protein fragment. We then devised INCYPRO to demonstrate that these types of cross-linkers can also stabilise the entire protein,” notes Grossmann. Robust and efficient enzymes are viable alternatives to toxic reagents used in the chemical and pharmaceutical industries, and often shorten the multi-step chemical/drug synthesis routes. “Straightforward and high-efficiency stabilisation approaches such as INCYPRO make a positive impact on the field of green chemistry,” concludes Grossmann.