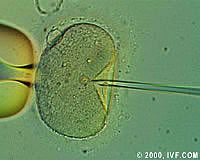
Parliament rejects move to ban human cloning
On 29 November the European parliament rejected a move to ban human cloning in the EU by 316 votes to 37. Italian MEP Francesco Fiori, who moved the failed resolution, called for new legislation. Defending his report, he said it was essential to decide now which doors must never be opened by our increasing knowledge of human genetics. The move follows an intensification of the debate over human cloning following the announcement by US company Advanced Cell Technology that they have successfully cloned an early-stage human embryo. During the debate, a split emerged between MEPs who support research into therapeutic cloning and those who totally oppose the use of human embryos for research purposes. Some scientists want to use therapeutic cloning to produce embryos which can be used to harvest stem cells, a potential source of cures for diseases such as multiple sclerosis and Alzheimer's. German MEP Hiltrud Beyer spoke out against therapeutic cloning on the grounds that to create human embryos and then destroy them would show a lack of respect for human life. 'We do not want a society where women's bodies are used to create embryos for profit,' she said. Irish MEP Dana Rosemary Scallon said she opposed even the limited research on human embryos which has already been approved by Parliament under the Sixth Framework programme, (FP6) for research and technological development (RTD). The Commission has said it will not finance research into human cloning, but would support stem cell research involving the use of aborted foetuses or surplus foetuses left over from test-tube fertilisation. Finnish MEP Astrid Thors, however, took the view that no doors should be closed, adding that she would like to have seen support for therapeutic cloning within FP6. MEP Gianfranco Dell'Alba, from Italy, also called for more research in the area of therapeutic cloning, explaining that 'research can give hope.' He said 'Yes to research on stem cells, yes to supernumerary embryos and yes to therapeutic research, which has nothing to do with cloning.' Scottish MEP John Purvis urged the Commission not to let European research fall behind that of other countries. He advocated using supernumerary embryos, left over from IVF treatment, to continue work in this area. Research Commissioner Philippe Busquin clarified the Commission's position on human genetics. He said the Commission intends to set up a group of scientific and legal experts in order to prepare the annual reports to the Parliament and the Council on Directive 98/44/EC on the patentability of biotechnological inventions. He said that he did not intend to grant Community funding to research on embryo stem cells if it lead to the production of human embryos purely for the purposes of research or stem cell production. Mr Busquin also called for clarification of Directive 98/44/EC, which he urged Member States to transpose and implement effectively. 'In a context of fierce international competition,' he said, 'the European Directive provides European researchers and industrialists with an indispensable common frame of reference. This is not the time to amend the directive or review its contents. However, some of its aspects require clarification. Science moves ahead at great speed, knowledge is expanding at an increasing rate, and there is a need for legislation.' He said that this legislation was the responsibility of political decision-makers and those in public office, as 'the courts cannot operate in a scientific vacuum, nor can science develop in a legal vacuum.' A Commission spokeswoman has said that there are as yet no legal restrictions on experiments that could lead to human cloning in seven EU Member States: Denmark, Finland, France, Luxembourg, the Netherlands, Portugal and Sweden. Candidate State Cyprus has introduced a hasty ban on human cloning, which was approved just 30 minutes after being presented in the country's Parliament, to stop one of its doctors from producing a human clone. Dr Panayiotis Zavos said he was planning to go through with the procedure 'by the end of the year.' Meanwhile, the UK is rushing through legislation which will ban reproductive cloning but not therapeutic cloning, which will be permitted for strictly controlled research purposes. The European Parliament debate followed a report on the ethical, legal, economic and social implications of human genetics drawn up by the Parliament's committee on human genetics and other new technologies in modern medicine. The committee was set up following a number of controversial developments including the birth in summer 1996 of 'Dolly the sheep' at the Roslin Institute, Scotland.